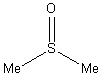
[67-68-5]
| Molecular formula | C2H6OS
 |
| Relative molecular mass | 78.13 |
| CAS registry |
[67-68-5] |
| Melting point | 18.5°C |
| Boiling point | 189°C |
Data taken from the Dictionary of Organic Compounds
DMSO is a clear, colourless, hygroscopic liquid. It is a dipolar aprotic solvent, miscible with water and soluble in many polar organic solvents such as alcohols, ester, ketones and chlorinated solvents. It will dissolve many inorganic salts.
Its ability to penetrate the skin is due to the fact that the molecule is highly polar, but also has two methyl groups which interact strongly with lipids in the skin. One particular danger associated with DMSO is that although not considered toxic itself, it is highly effective at transferring other (potentially toxic) substances into the body via skin contact. For example skin contact with DMSO and a cyanide salt would pose a high risk of cyanide poisoning. DMSO will dissolve and penetrate ordinary rubber gloves, so alternative materials should be used such as butyl rubber or blue nitrile.
Other safety concerns associated with DMSO are that it is irritant and harmful. Prolonged exposure can lead to dermatitis, and possibly to liver and kidney damage. It can produce explosive reactions with some compounds, such as acid chlorides.