The Haber Process
 The commercial synthesis of ammonia began, not with the peaceful use of fertilizer, but with the necessities of war. In the early years of this century, Germany understood that any war that it might have with England would, at least initially, result in the blockade of critical war materials from abroad. The most important of these resources was quano, manure from seagulls that roosted along the coast of Chile. This quano was rich in nitrates and was the basis of the German manufacture of explosives. The problem was that it had to be shipped by the tanker-load across the Atlantic and past patrolling British warships.
The commercial synthesis of ammonia began, not with the peaceful use of fertilizer, but with the necessities of war. In the early years of this century, Germany understood that any war that it might have with England would, at least initially, result in the blockade of critical war materials from abroad. The most important of these resources was quano, manure from seagulls that roosted along the coast of Chile. This quano was rich in nitrates and was the basis of the German manufacture of explosives. The problem was that it had to be shipped by the tanker-load across the Atlantic and past patrolling British warships.
But during this period, the chemistry of ammonia synthesis was being explored by the German chemists Fritz Haber and Walther Bosch who found that it was possible to produce ammonia from nitrogen and hydrogen by the process:
N2 + 3 H2 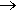 2 NH3
2 NH3
 Previously the problem had been that N2 is a very stable molecule, and so most attempts to convert it to less stable molecules, such as NH3, failed because of thermodynamic or entropy problems. The secret to the Haber-Bosch process proved to be a catalyst of iron with a small amount of aluminium added (aluminium was at the time an exotic and expensive metal that probably attracted Haber's attention as a novelty). The Haber-Bosch process operates at high pressure so as to shift the equilibrium to the right, and high temperature to increase the rates of the reaction. Of course, operating at high temperature actually shifted the reaction to the left, but the trade-off for faster rates was accepted. By removing the ammonia as liquid ammonia, the equilibrium is continuously shifted to the right.
Previously the problem had been that N2 is a very stable molecule, and so most attempts to convert it to less stable molecules, such as NH3, failed because of thermodynamic or entropy problems. The secret to the Haber-Bosch process proved to be a catalyst of iron with a small amount of aluminium added (aluminium was at the time an exotic and expensive metal that probably attracted Haber's attention as a novelty). The Haber-Bosch process operates at high pressure so as to shift the equilibrium to the right, and high temperature to increase the rates of the reaction. Of course, operating at high temperature actually shifted the reaction to the left, but the trade-off for faster rates was accepted. By removing the ammonia as liquid ammonia, the equilibrium is continuously shifted to the right.
By 1913, the German chemical giant BASF (Badashe Analine und Soda Fabrik) had a plant operating in Ludwigshaven-Oppau, Germany making ammonia at the rate of 30 metric tons per day. Without question, this technology permitted Germany to continue making explosives and extended the war for many years.
- Agricultural uses: NH3 is used to make NH4NO3, which is an important fertiliser, and helped to ensure the western world could grow enough food on its limited farmland areas.
- Explosives: NH3 is oxidised to NO2 and NO3, which is then dissolved in water to make nitric acid. HNO3 is the main starting reagent for most nitro-based explosives including TNT, RDX and Semtex.
Interesting Notes on Haber:
 He was a fanatical patriot, and thought that a scientist should do everything in their power to help their country, especially in time of war. When WW1 broke out, he had the idea to use poison gases to kill troops in the trenches, and thus break the stalemate on the Western Front. As such, he was effectively the Father of Chemical warfare. He first used chlorine gas in 1915 at the battle of Ypres, where it took the unsuspecting (and unprotected) French troops by surprise - killing over 10,000 of them in in few minutes. Over the next few years he developed other more lethal and nasty gases, such as phosgene and finally mustard gas, all of which were used against Allied troops.
He was a fanatical patriot, and thought that a scientist should do everything in their power to help their country, especially in time of war. When WW1 broke out, he had the idea to use poison gases to kill troops in the trenches, and thus break the stalemate on the Western Front. As such, he was effectively the Father of Chemical warfare. He first used chlorine gas in 1915 at the battle of Ypres, where it took the unsuspecting (and unprotected) French troops by surprise - killing over 10,000 of them in in few minutes. Over the next few years he developed other more lethal and nasty gases, such as phosgene and finally mustard gas, all of which were used against Allied troops.
His wife (also a chemist) objected so much to his 'immoral' role that she committed suicide by shooting herself in his living room. The very next day Haber went to the Eastern Front to continue his gas tests...
Haber received the Nobel prize in 1918 for his ammonia process and 'contributions to agriculture', but because of his association with chemical weapons, there were many objections to him receiving the prize. In fact he was regarded as a war criminal by the Allies, but was never prosecuted, probably due to the political embarrassment it would have caused to prosecute a Nobel prize winner for war crimes!
After the war he continued to make poison gases for the German government under the cover of projects to make insecticides. One gas he invented was a cyanide-based gas named Zyklon B. This is quite ironic, since Haber was originally of Jewish descent (although he renounced his religion in favour of Christianity to 'appear more German') - he didn't realise that this gas was to be used 15 years later in the Nazi death camps to kill his Jewish countrymen.
When the Nazis came to power in the early 1930s, they treated him with suspicion because of his Jewish heritage, and when they started deporting his Jewish technicians, Haber decided he could no longer remain in the country he loved, and so fled to Switzerland. He remained there in self-imposed exile until his death some years later. Again, the irony was that the supreme patriot, who had done so much to help the German war efforts, was abandoned by his own country.
 More information about Haber from the Nobel Institute.
More information about Haber from the Nobel Institute.
Paul May, 1999
 The commercial synthesis of ammonia began, not with the peaceful use of fertilizer, but with the necessities of war. In the early years of this century, Germany understood that any war that it might have with England would, at least initially, result in the blockade of critical war materials from abroad. The most important of these resources was quano, manure from seagulls that roosted along the coast of Chile. This quano was rich in nitrates and was the basis of the German manufacture of explosives. The problem was that it had to be shipped by the tanker-load across the Atlantic and past patrolling British warships.
The commercial synthesis of ammonia began, not with the peaceful use of fertilizer, but with the necessities of war. In the early years of this century, Germany understood that any war that it might have with England would, at least initially, result in the blockade of critical war materials from abroad. The most important of these resources was quano, manure from seagulls that roosted along the coast of Chile. This quano was rich in nitrates and was the basis of the German manufacture of explosives. The problem was that it had to be shipped by the tanker-load across the Atlantic and past patrolling British warships. Previously the problem had been that N2 is a very stable molecule, and so most attempts to convert it to less stable molecules, such as NH3, failed because of thermodynamic or entropy problems. The secret to the Haber-Bosch process proved to be a catalyst of iron with a small amount of aluminium added (aluminium was at the time an exotic and expensive metal that probably attracted Haber's attention as a novelty). The Haber-Bosch process operates at high pressure so as to shift the equilibrium to the right, and high temperature to increase the rates of the reaction. Of course, operating at high temperature actually shifted the reaction to the left, but the trade-off for faster rates was accepted. By removing the ammonia as liquid ammonia, the equilibrium is continuously shifted to the right.
Previously the problem had been that N2 is a very stable molecule, and so most attempts to convert it to less stable molecules, such as NH3, failed because of thermodynamic or entropy problems. The secret to the Haber-Bosch process proved to be a catalyst of iron with a small amount of aluminium added (aluminium was at the time an exotic and expensive metal that probably attracted Haber's attention as a novelty). The Haber-Bosch process operates at high pressure so as to shift the equilibrium to the right, and high temperature to increase the rates of the reaction. Of course, operating at high temperature actually shifted the reaction to the left, but the trade-off for faster rates was accepted. By removing the ammonia as liquid ammonia, the equilibrium is continuously shifted to the right. He was a fanatical patriot, and thought that a scientist should do everything in their power to help their country, especially in time of war. When WW1 broke out, he had the idea to use poison gases to kill troops in the trenches, and thus break the stalemate on the Western Front. As such, he was effectively the Father of Chemical warfare. He first used chlorine gas in 1915 at the battle of Ypres, where it took the unsuspecting (and unprotected) French troops by surprise - killing over 10,000 of them in in few minutes. Over the next few years he developed other more lethal and nasty gases, such as phosgene and finally mustard gas, all of which were used against Allied troops.
He was a fanatical patriot, and thought that a scientist should do everything in their power to help their country, especially in time of war. When WW1 broke out, he had the idea to use poison gases to kill troops in the trenches, and thus break the stalemate on the Western Front. As such, he was effectively the Father of Chemical warfare. He first used chlorine gas in 1915 at the battle of Ypres, where it took the unsuspecting (and unprotected) French troops by surprise - killing over 10,000 of them in in few minutes. Over the next few years he developed other more lethal and nasty gases, such as phosgene and finally mustard gas, all of which were used against Allied troops.