
AZT (Zidovudine)
The first antiretroviral medication approved for treating HIV.

Henry Goss-Custard
Eton College

Molecule of the Month April 2025
Also available: HTML version.

|

UK government AIDS awareness campaign TV bulletin and leaflet from 1987.
[Image: Crown Copyright via Wikimedia Commons] |
What is it called AZT?
AZT is the abbreviation for azidothymidine, although it is sold under its trade name of Zidovudine. It was the first antiretroviral medication developed and approved for managing and treating HIV.
 |
 |
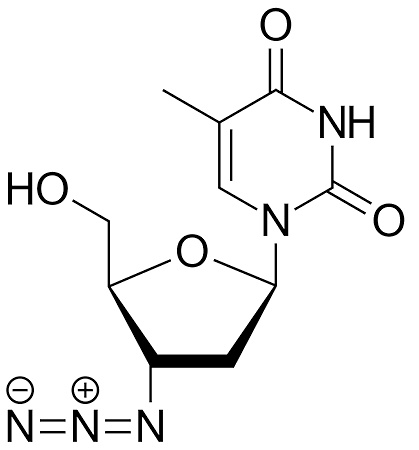
AZT (azidothymidine)
[Image: Fvasconcellos, Public domain, via Wikimedia Commons] |
Spacefill model of AZT |
How was it discovered?
In the early 1960s, researchers synthesised AZT as a potential treatment for cancer, operating under the hypothesis that certain cancers were caused by viruses. They aimed to develop antiviral agents that could inhibit these viruses and thus prevent cancer development. But initial trials in mice had unpromising results, leading to the abandonment of AZT. About a decade later, however, German researchers discovered that AZT effectively inhibited the 'Friend' virus, which is a leukaemia retrovirus that causes cancer in mice, thereby demonstrating it had antiviral efficacy.
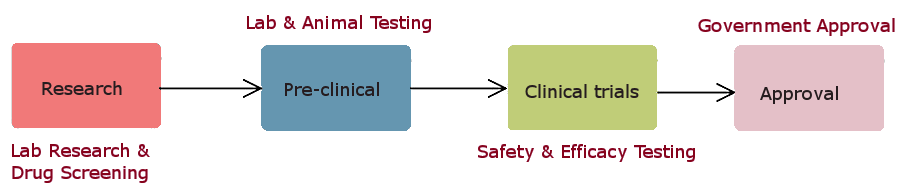
It takes many years, sometimes decades, from the initial research on a drug like AZT until it is finally approved for use by doctors.
What's a retrovirus?
They are viruses that insert a DNA copy of their own RNA genome into the host cell's DNA. The host cell is then turned into a 'factory' whose sole job is to churn out new copies of the retrovirus, which go on to infect more cells.
Ok, so what happened next?
Unfortunately, further studies were not conducted because retroviruses were not yet known to cause any human diseases. It wasn’t until the mid-1980s, a few years after the first reported case of AIDS, that the antiretroviral properties of AZT were recognised as being potentially useful in human medicine.
At a time when treatment options were desperately limited, AZT offered a lifeline to clinicians treating AIDS, despite its association with a range of unpleasant side-effects. These side-effects were considered an acceptable trade-off given the drug’s ability to extend life and to manage the disease. However, as newer, more advanced treatments have since become available, offering similar or greater benefits with significantly fewer risks, AZT is generally no longer the preferred option.
So what is the history of HIV and AIDS - why was AZT needed?
In 1981, a series of unusual illnesses began surfacing in the United States, the first clinical signs of what would later be recognised as AIDS. These cases primarily emerged among injection-drug users and gay men, both of whom were experiencing severe immune system deficiencies. With a compromised immune system, the victims often suffered and even died from a range of unusual other illnesses, many of which were rarely severe, let alone fatal, in 'normal' patients. Among these illnesses were pneumocystis jirovecii pneumonia (PJP) and Kaposi's sarcoma (KS), a cancer that had previously been rare in the population. The rising number of PJP and KS cases prompted the Centers for Disease Control and Prevention (CDC) to establish a task force to monitor and investigate this emerging public health threat.
Initially, the CDC named the disease based on the specific conditions it induced. Along the way, other names such as GRID (Gay-Related Immune Deficiency)—reflecting its prevalence among gay men—and ‘4H disease’ (referring to homosexuals, heroin users, haemophiliacs, and Haitians) were briefly in use. However, as it became apparent that the disease was affecting a broader population, the terminology was revised. By July 1982, the name ‘AIDS’ (Acquired Immune Deficiency Syndrome) was proposed and officially adopted.
 |
 |
Poster from the Freddie Mercury tribute concert in 1992.
Freddie died of AIDS the year before and the concert was used
to spread awareness about the dangers of AIDS. |
US AIDS awaremess poster from the 1980s
showing that HIV is often symptomless.
[Wellcome Images via Wikimedia Commons] |
In the 1980s and early 1990s there was a lot of worry about the new incurable, potentially fatal, epidemic of AIDS, and governments everywhere made posters and flyers to promote awareness of the disease and how to prevent its spread.
And I heard that many famous people died from it...?
Yes, as the death toll mounted, HIV inevitably started infecting famous people from the world of sports, music, cinema and politics. Many of these subsequently died of AIDS-related illnesses, and these high profile deaths really pushed the topic into the mainstream agenda, creating a huge demand for a cure for this new deadly disease.
 |
 |
 |
 |
Rock Hudson
Hollywood movie star
[Image: Phil Stern (1919-2014),
Public domain, via Wikimedia Commons] |
Kenny Everett
UK TV and radio comedian
[Image: Wikimedia Commons] |
Liberace
Pianist and entertainer
[Image: Allan warren,
CC BY-SA 3.0 via Wikimedia Commons] |
Freddie Mercury
Lead singer of rock group Queen
[Image: Carl Lender,
CC BY-SA 3.0 via Wikimedia Commons] |
Some of the many famous people diagnosed with HIV who subsequently died of AIDS-related illnesses.
So what was causing AIDS?
Amid the urgent search to uncover the cause of AIDS, two independent research teams were closing in on a breakthrough. One team, led by Drs Luc Montagnier and Françoise Barré-Sinoussi at the Pasteur Institute in France, studied patients who showed early symptoms of AIDS, such as swollen lymph nodes and persistent fatigue. They managed to isolate a new virus from these patients, which they named lymphadenopathy-associated virus (LAV).
Meanwhile, in the United States, Dr Robert Gallo and his team were also investigating the cause of AIDS. They identified a virus they called HTLV-III, thinking it was related to a family of viruses (HTLVs) they had previously discovered that were linked to certain types of leukaemia. Initially, there was debate over whether LAV and HTLV-III were the same virus or different ones. Further research revealed that HTLV-III and LAV were, in fact, the same virus, and they were collectively renamed HIV (human immunodeficiency virus) in 1986.
 |
 |
 |
Dr Luc Montagnier
Jointly won the 2008 Nobel Prize in Physiology
or Medicine for the discovery of HIV.
[Image: Prolineserver, GFDL 1.2 via Wikimedia Commons] |
Dr Françoise Barré-Sinoussi
Jointly won the 2008 Nobel Prize in Physiology
or Medicine for the discovery of HIV.
[Image: Prolineserver, GFDL 1.2 via Wikimedia Commons] |
Dr Robert Gallo
Played a crucial role in establishing HIV as the infectious
agent responsible for AIDS and in the development of
the HIV blood test.
[Image: © 1995 Túrelio (via Wikimedia-Commons), 1995] |
So HIV is the name of the virus that causes AIDS?
Yes, that's correct.
How does HIV work?
HIV is a remarkable creation of nature, which follows a well-defined replication cycle that targets several of the the body's immune cells, specifically, its CD4+ Helper T cells, macrophages and antigen-presenting dendritic cells.
Wait, what are those?
Antigen-presenting dendritic cells (APDCs) are cells that capture invading foreign organisms and present them to the T cells to activate the immune system. CD4+ Helper T cells are a type of white blood cell. They are called 'helper cells' because they can identify infectious organisms (bacteria, viruses) and then send signals to other types of immune cells, called macrophages, which then destroy the infectious organism and remove dead cells.
Think of this like an invading army trying to capture a well-defended castle. The APDCs are like scouts that capture some of the invading soldiers, and take them back to the castle so that the commanders (T cells) can identify the invaders as being enemy soldiers. The commanders then raise the alarm flag, alerting the rest of the defending army (macrophages) in the castle that they should go out and kill all the enemy invaders they find.

A virus attack on a cell is like an enemy army attacking a castle.
The spotters (APDCs) on the castle roof alert the commanders to a possible attack.
The commanders (T cells) identify the approaching army (virus) as hostile,
and send a signal to the defending army (macrophages) to kill the invaders.
If any of these defending forces become depleted, i.e. the defending scouts, commanders or the army, are killed, the castle will be overrun and fall to the enemy. Similarly, if any of those immune cells are killed or their numbers are significantly reduced, the body becomes vulnerable to a wide range of infections that it would normally fight off. HIV is very efficient at killing these immune cells!
So how does HIV do this?
The HIV infection cycle involves several stages that enable the virus to hijack the host's cellular machinery, which it then uses to mass reproduce its own viral components. HIV replication is extremely destructive to the host cell, meaning that as the virus replicates and the infection develops, the immune system gradually degrades, ultimately resulting in AIDS.
The infection process of HIV starts when the virus attaches itself to specific receptors, called 'CD4 receptors', on the surface of a Helper T cell. This attachment is possible because the virus has 2 glycoproteins on its surface which consist of protein and carbohydrate chains that act like 'sticky fingers'. These stick to the CD4 receptor, which causes the receptor to change shape (‘conformational change’) — that reveals new sites on the cell, called CCR5, which the virus can bind to. The shape changes and the additional binding tether the virus more strongly to the cell, but importantly they also cause a 'back door' to be opened through the cell membrane to the inside of the cell. The HIV virus can now inject its genetic RNA material and viral proteins through the back door into the immune cell.
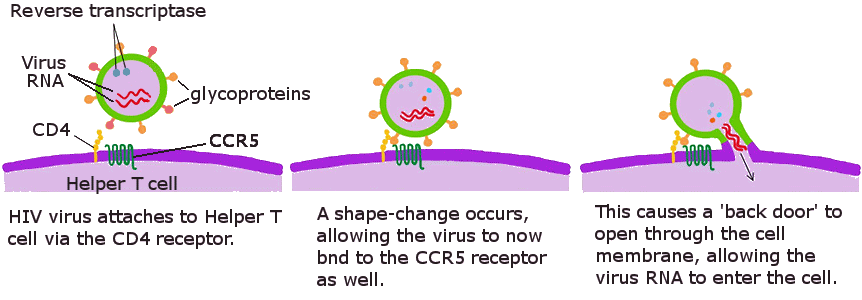
A simplified diagram showing how HIV infects a cell.
So it's game-over for the cell?
Pretty much. The virus' genetic material enters the host cell as single-stranded RNA, whch is one half of DNA. An enzyme called reverse transcriptase then adds the missing other half to the RNA molecule to make the new DNA, and this process is called 'reverse transcription'.
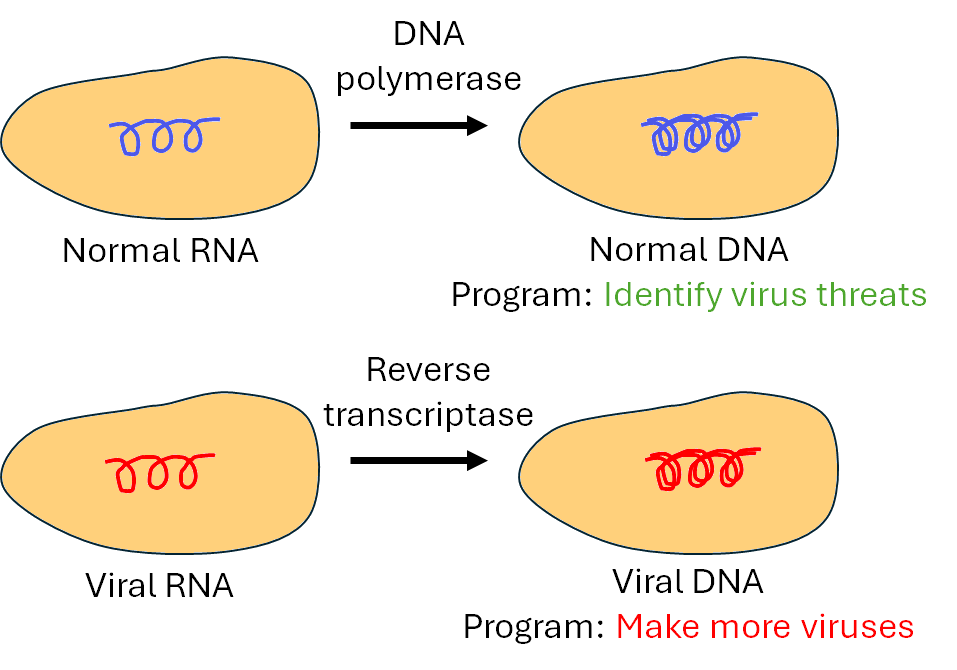
In the nucleus of a cell, single-stranded RNA is converted into double-stranded DNA using enzymes.
In a normal T Helper cell, the DNA is programmed to identify potential threats to the cell, such as viruses.
In a T Helper cell infected with HIV, the viral DNA now has a new program - to simply make more and more viruses.
In a normal (uninfected) cell, DNA is fabricated using DNA polymerase enzymes, which take the RNA template and copy it twice, and join the two complementary parts together to make DNA. Reverse transcriptase works in a similar way to this, however it lacks a proofreading mechanism, which is a quality control process that corrects errors during DNA synthesis. Without this ability to correct mistakes, errors, or mutations, accumulate in the viral DNA nearly a million times more frequently than in other cells that have proofreading mechanisms.
AZT specifically targets reverse transcriptase, and its importance will soon become evident.
Is the high mutation rate significant?
While many mutations will be inert or simply kill the virus, others can introduce subtle changes to the virus’s proteins, increasing genetic diversity, and giving rise to marginally different structures or enzymes. In fast-replicating viruses like HIV, this diversity makes developing medical treatments more challenging because it allows the virus to rapidly adapt to these medical treatments. Although many drugs are initially effective, ongoing mutations enable the virus to develop resistance to them over time, even within a single patient, requiring treatments to evolve in response.
Once an infection is established, HIV replication produces about ten billion infected cells per day, sometimes resulting in numerous variants within the same infected patient in the course of just one day.
To tackle the high mutation rate of HIV and resulting drug resistance, combination antiretroviral therapy — often involving multiple drugs — is utilised. This approach is known as Highly Active Antiretroviral Therapy and employs a selection of medications that target different stages of the HIV life cycle. This way, it becomes significantly more difficult for the virus to mutate in ways that render all treatments ineffective.
Back to the life cycle - how is viral DNA integrated into the host?
Once HIV’s double-stranded DNA is formed, it is transported into the host cell’s nucleus where another enzyme, called integrase, precisely cuts the host DNA and permanently joins the viral DNA to it via a covalent bond. This integration embeds the viral genome into the host’s genetic material, so each time the host cell divides, it replicates the viral DNA along with its own, allowing the virus to persist indefinitely within the body.
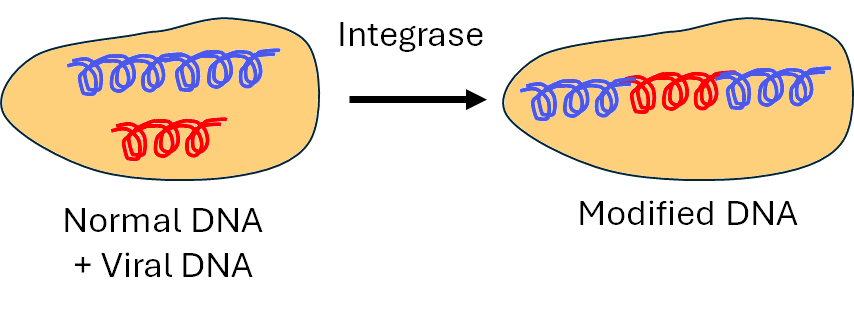
The viral DNA is inserted into the normal cell DNA, reprogramming the cell to become a 'virus factory'.
A defining feature of HIV’s incurability is this integration into a long-lasting ‘latent reservoir’ of infected cells, where the virus’s genetic material lies dormant, hidden from immune detection and inaccessible to antiretroviral therapies within the genome of the cell itself. The virus can remain in this hidden state for months or even years before reactivating, leading to new rounds of viral replication and illness. By driving some of the cells it infects into a latent state, HIV ensures that these cells can migrate to tissues rich in additional target cells before reactivation. This strategic latency maximises the virus’s chances of spreading to new cells and sustaining the infection long-term.
Ok - but how does HIV cause AIDS?
A patient is considered to have AIDS when their CD4 count drops below 200 cells per cubic millimetre (an uninfected person typically has a count of between 500-1400 cells per cubic millimetre). Unlike many other viral infections, it is not the HIV virus itself that directly causes the fatal outcomes. Instead, as Helper T cell numbers continue to decline, the immune system becomes severely weakened, leaving the body vulnerable to opportunistic infections and cancers that a healthy immune system would typically fend off easily. It is this loss of immune function, rather than the virus itself, that eventually leads to AIDS.
Time for the cavalry to come to the rescue - how does AZT enter the cell?
AZT is a small moecule that is lipophilic, which means it dissolves in fats, oils, lipids and non-polar solvents. It enters the host cell primarily through passive diffusion through the fatty cell membrane.
Ok, but how does AZT actually work?
AZT works in the same way as many other drugs, blocking key enzymes or proteins and preventing them from performing their intended functions. While HIV's replication process is remarkably sophisticated and efficient, it harbours one major vulnerability that can be exploited. AZT inhibits the viral reverse transcriptase enzyme, and therefore prevents the conversion of HIV’s RNA into DNA. As a result of this, the integration of viral DNA into the host cell cannot occur, meaning that the replication of the virus is halted.
AZT closely resembles the natural nucleoside thymidine, one of the four building blocks of DNA. However, AZT has a key structural difference: it contains an azido group (–N3) instead of a hydroxyl group (–OH) at the 3' position of the deoxyribose sugar.
 |
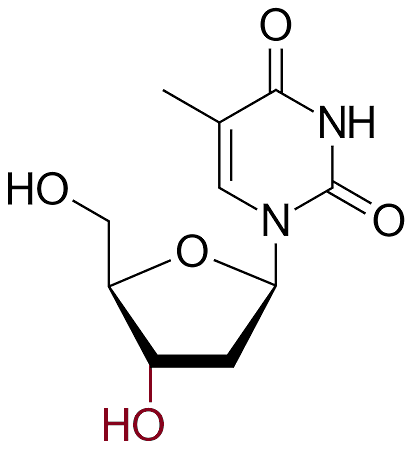 |
| AZT |
Thymidine
(The 3' hydroxy group is shown in red). |
|
|
Once inside the host cell, AZT is initially inactive and must undergo phosphorylation (the addition of a phosphoryl group) to become active. Cellular enzymes sequentially add three phosphate groups to AZT in a series of steps, converting it into AZT monophosphate (AZT-MP), then AZT diphosphate (AZT-DP), and finally into AZT triphosphate (AZT-TP), the active form of the drug.
When reverse transcriptase is synthesising viral DNA, it cannot distinguish between thymidine triphosphate and AZT-TP due to their structural similarity. As a result, AZT-TP is incorporated into the new DNA chain in place of thymidine. However, because AZT-TP lacks the 3' hydroxyl group necessary for bonding with the next part of the DNA molecule, the DNA chain elongation is prematurely terminated. An analogy would be to ask a group of people to link hands to form a chain, which works well until the chain gets to a person that has only has one arm - then the chain stops at that point. With the DNA incomplete, the cell can no longer fabricate HIV proteins, and the virus reproduction process stops.
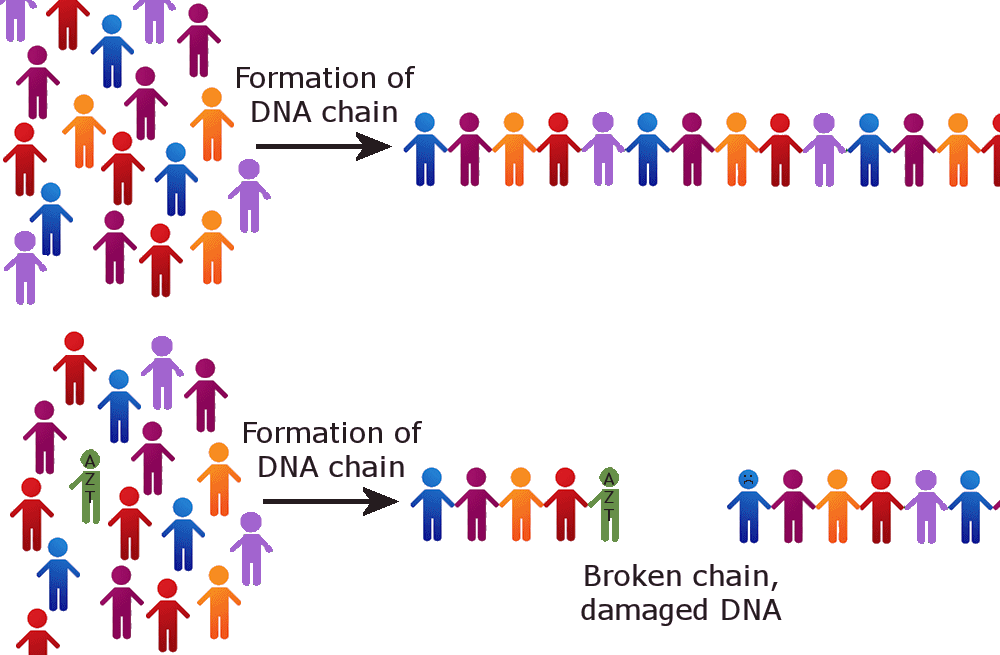
DNA is like a long chain of molecules, like people holding hands in a line. AZT is like a person with one hand - they break the chain and damage the DNA, killing the cell and preventing the spread of the virus.
Clever. So, how is AZT chemically synthesised?
The synthesis is shown in the figure, and begins with thymidine, which consists of a pyrimidine base (thymine), linked to a sugar. The sugar has two hydroxyl groups: the primary 5′-OH and the secondary 3′-OH. The 3′-OH is the target for modification, so the 5′-OH is protected with a bulky triphenylmethyl (trityl) group to ensure selective reactions and prevent side reactions.
Next, the 3′-OH is converted into a methanesulfonate (mesylate, –OSO2CH3) group using mesyl chloride (MeSO2Cl). The mesylate is an excellent leaving group, as the resonance stabilisation of the sulfonate anion (OSO2-) makes it highly stable when displaced. Reacting this with a base (B) converts the compound into anhydrothymidine, where the 3′-carbon position is now a highly reactive site, primed for nucleophilic attack.
An azide ion (N3-) is used to attack this reactive 3′-carbon position, replacing the original 3′-OH group with an azido group (–N3-) to give the final product, AZT.
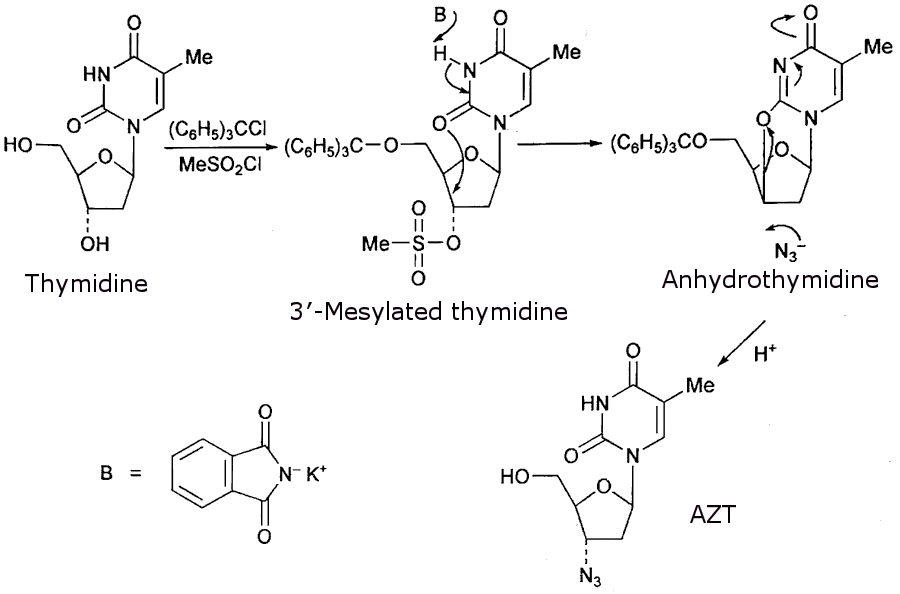
Synthesis of AZT.
If AZT inhibits viral DNA reproduction, won’t it also interfere with human DNA reproduction?
Yes, at elevated concentrations, the active triphosphate form of AZT may begin to inhibit human cellular DNA polymerases as well! However, the drug exhibits a markedly higher affinity for HIV's reverse transcriptase (approximately 100 times greater) making it far more selective for viral rather than human replication mechanisms. This preference is likely attributed to the human cells' ability to repair interruptions in their DNA sequence when affected by AZT, a mechanism not present in the HIV virus.
Yet, at some (high-dose) threshold, AZT can also start to inhibit mitochondrial DNA polymerase, adversely affecting mitochondrial DNA replication. This inhibition explains AZT's reversible toxicity observed in some patients, especially in tissues with high energy demands, such as cardiac and skeletal muscle, where mitochondrial impairment can lead to symptoms like myositis (a condition where the immune system attacks the muscles).
Are there any alternatives to AZT? Is it still commonly used today?
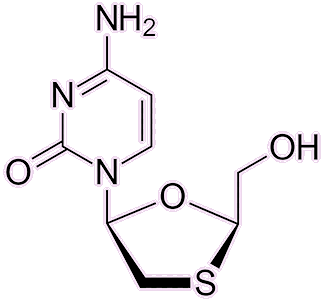
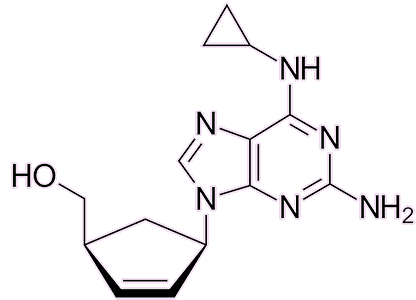
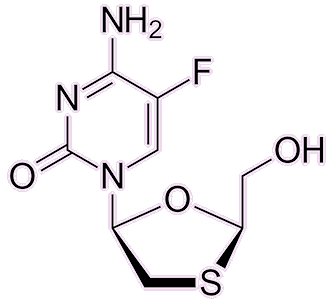
AZT is no longer the default medication for HIV: several better alternatives have since emerged. Medications like lamivudine, emtricitabine, abacavir, and tenofovir disoproxil — all reverse-transcriptase inhibitors — have gained favour due to their improved efficacy and fewer side-effects. AZT’s rather serious side-effects, including headaches, fatigue, nausea and even bone-marrow suppression, reduce its widespread use today.
 |
 |
| Lamivudine |
Abacavir |
|
|
 |
 |
| Emtricitabine |
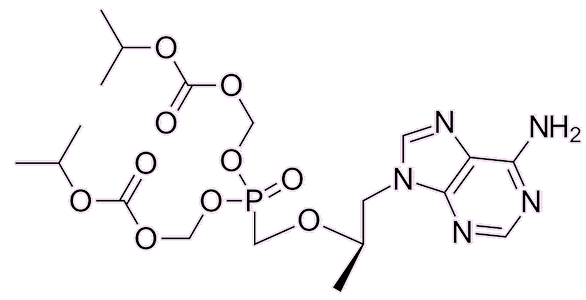
Tenofovir disoproxil |
|
|
Other classes of antiretroviral agents now also exist, including protease inhibitors, integrase inhibitors, and entry inhibitors (each referring to the specific stage of the HIV replication cycle which the drug seeks to disrupt), and offer more strategies to treat the infection. These advancements have led to combination therapies that enhance effectiveness and simplify treatment regimens, improving patient adherence.
Whilst AZT may no longer be the first-line therapy, it still remains important in specific contexts, such as preventing mother-to-child transmission during childbirth or in parts of the world where newer medications are less widely available, such as in parts of Africa.

Bibliography
- Matt Carter (n.d.). Reverse Transcription - an overview, ScienceDirect Topics.
- Wikipedia. (2020), Zidovudine.
- Animated HIV Science (2019), YouTube, HIV Life Cycle.
- Wikipedia. (2021), HIV.
- R.S. Vardanyan (n.d.), Zidovudine - an overview, ScienceDirect Topics.
- The Science Snail, (2019), AZT – mechanism of action and organic synthesis.
- Maugh, T.H. (1994), HIV Researchers Struggle to Hit Their Moving Target: Disease: The virus frustrates scientists with its abili, Los Angeles Times.


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.28204721]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.28204721]
![]()
![]()
![]()
![]()























