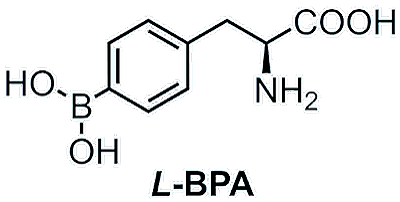
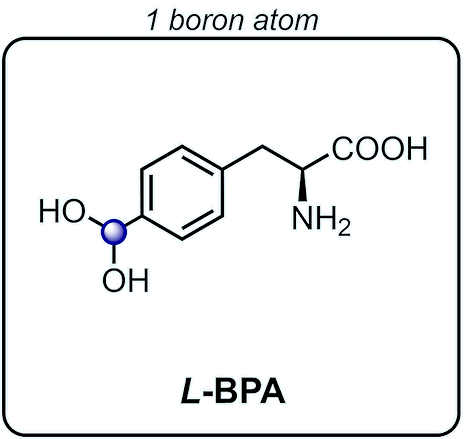
BPA
(L-4-Boronophenylalanine)
An unnatural amino acid used in
boron neutron capture therapy for combating cancer

Dane Jemc
University of Ljubljana, Slovenia

Molecule of the Month November 2025
Also available: HTML version.

|
|
BPA... isn’t that something to do with plastic?
If you’ve come across scary headlines about toxic plastics, you’ve definitely met BPA or Bisphenol A (see the MOTM for August 2013). But the BPA we are talking about here is a totally different molecule.
Boronophenylalanine, or BPA for short, is an amino acid, specifically an unnatural one. It is used for an innovative and modern method of cancer treatment.
Wait – what is an unnatural amino acid?
Perhaps you’ve heard of the 20 well-known alpha amino acids that make up natural proteins – alanine, glycine, phenylalanine, etc. Let’s see what they are chemically.
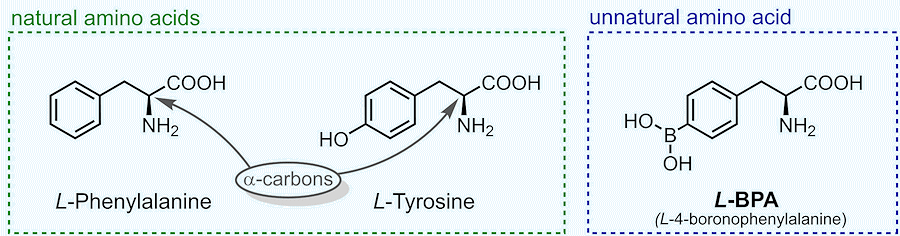
Comparison of the structures of BPA and other alpha amino acids.
[Image: self-drawn]
|
Amino acids are like Lego bricks of life, with each one containing two special chemical groups: an amino group (-NH2) and a carboxyl group (-COOH). If these two are attached to the same carbon atom, we call this Lego brick an alpha amino acid.
The famous 20 alpha amino acids that were mentioned are natural. But why? They get their name from the fact that we find them in nature AND that they can make up natural protein chains. All other amino acids are referred to as unnatural. This includes all synthetically made amino acids, as well as other amino acids found in nature that lack the ability to form proteins.
If we look at the structure of BPA above, we can classify it as an alpha amino acid, and can see it looks suspiciously similar to either phenylalanine or tyrosine (this similarity will matter when we try synthesizing BPA).
|

Some natural amino acids are sold as food supplements.
[Image: Sandstein, Public domain via Wikimedia Commons]
|
So, how can BPA kill cancer?
On its own, BPA is kind of boring. It doesn’t poison cells or emit any radiation that could destroy cancer. It just travels around the body. Nothing deadly about it.
However, if you bombard BPA (containing atoms of boron-10) with neutrons, a nuclear reaction takes place, as depicted on the scheme on the right. When boron from BPA captures a neutron, it converts 'explosively' into a lithium ion and a helium ion (alpha particle). These tiny ion bullets can hit DNA and damage it, which causes the unlucky cell to die.
As you can probably imagine, it would be ideal if we could get boron-10 just into a tumour, shoot some neutrons at it, and – just like that – kill only the cancer cells. Well, that’s the main idea behind boron-neutron-capture therapy, or BNCT, which was first proposed almost a century ago
But doesn’t all that radiation also harm other cells?
Luckily, alpha radiation (helium ions) and lithium ions can’t travel far in animal tissues. Alpha particles penetrate only about 10 micrometres – shorter than the diameter of a single cell (10–100 micrometres). Lithium ions go even less far. This ensures that only the cell containing boron-10 gets damaged, while the neighbouring cells remain safe.
|
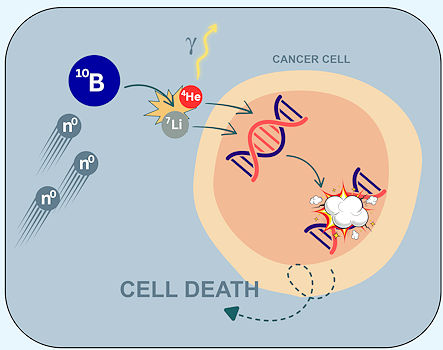
Principle of BNCT induced cell death.
[Image: self-drawn]
|
Surprisingly, low-energy neutrons used for BNCT aren’t like X-rays and cannot damage DNA on their own. They pass harmlessly through the body, unless they happen to encounter a partner in crime, a boron-10 atom. When the duo is united, they become lethal to whatever cell the B atom happens to be inside.
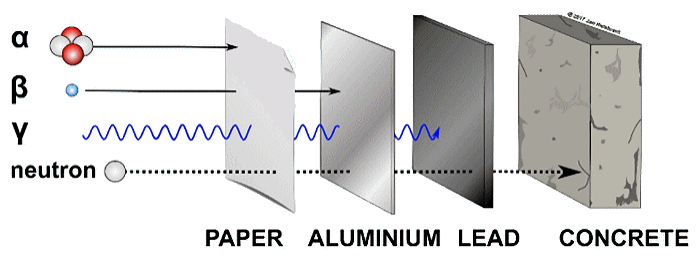
Penetrating power of different types of radiation.
[Image: Juhele, Public domain via Wikimedia Commons]
Why boron of all elements?

Elemental boron.
[Image: James St. John, Public domain via Wikimedia Commons] |
Boron is a very special element. It exists in nature as a mixture of two isotopes: 20% of boron-10 and 80% of boron-11. As you would expect, the interesting isotope is the naturally less abundant one.
Now, let’s review all the good characteristics, that make boron-10 viable for BNCT:
- Firstly, boron-10 is like an atomic piñata. Hit it with a neutron and it bursts, spitting out an alpha particle and a lithium ion. These can smash DNA to pieces which is ideal for cancer therapy as it kills cells.
- Secondly, boron is like a neutron sponge. It is one of the few elements that absorbs neutrons exceptionally well. Technically, we say it has a neutron absorption cross-section of 3840 barns, which is a hundred- or even a thousand-fold larger than that of the other elements in the human body. If other elements, like carbon or oxygen, behaved in the same way, BNCT would be impossible because the neutrons would be absorbed all over the place, risking tissue damage and not reaching the boron.
- Thirdly, boron is present in our body only in trace amounts. If a boron-containing molecule is injected into the body, it will be (approximately) the only molecule that absorbs neutrons well. That means sneaking it solely into tumours would leave healthy tissues mostly untouched during BNCT.
|
And how do we sneak BPA into tumours exactly?
It is all done by knowing the tumour very well. Tumours are usually quite hungry. They want to grow fast, so they need a lot of nutrients including amino acids. To harvest the body’s amino acids, the cancer cell makes more channels through which amino acids can pass inside. In scientific terms, we say: 'Cancer cells overexpress L-amino acid transporters'. Consequently, amino acids present in the bloodstream are taken up into tumour cells significantly more than into healthy tissues. (Un)fortunately, not all amino acids end up in the tumour, which in BPA’s case means some damage to healthy cells does occur during BNCT. The tumour-to-blood concentration ratio for BPA is at most 3.5 to 1.
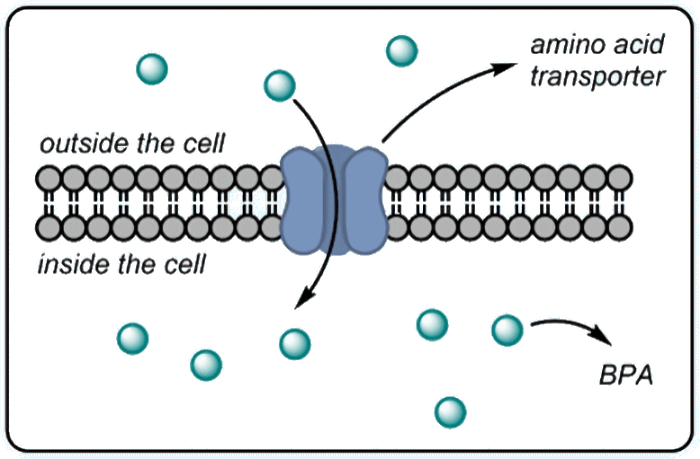
The mechanism of tumour uptake of BPA.
[Image: self-drawn]
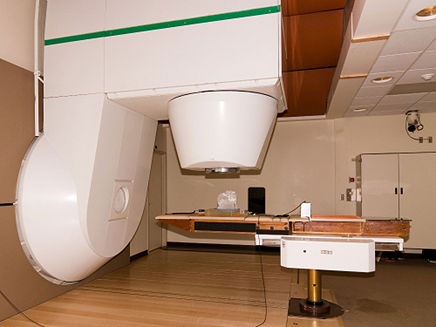
Is BNCT even being used?
BNCT is being used, but only to a limited extent. Japan leads with a few clinics using it commercially, while facilities in Finland, China, Argentina, and other countries are conducting clinical or pre-clinical trials on BNCT.
The reasons behind the lack of widespread use lie in two crucial aspects of the therapy: boron delivery to the tumour and irradiation with neutrons.
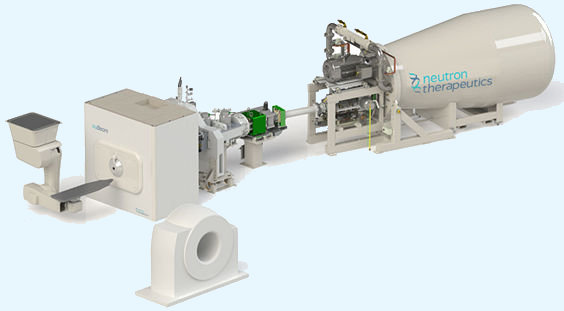
For decades, the problem was that neutrons couldn’t be generated with convenient, compact machines. A literal nuclear reactor in your hospital basement was required – not exactly patient- or hospital-friendly! Only recently have scientists figured out a way to make compact, accelerator-based neutron sources, making BNCT technology much more accessible to medical facilities.
On the other hand, boron delivery is problematic as well. The challenge is getting boron solely to cancer cells in a large enough amount.
BPA only has one boron atom per molecule. When it accumulates in tumours, lots of boron is required for the therapy to be effective; unfortunately, BPA usually can’t provide an adequate amount.
There is another compound used for BNCT called sodium borocaptate (BSH). This one contains a lot more boron, but it doesn’t stay in tumours for very long. That’s why BPA is thought to be the better option of the two.
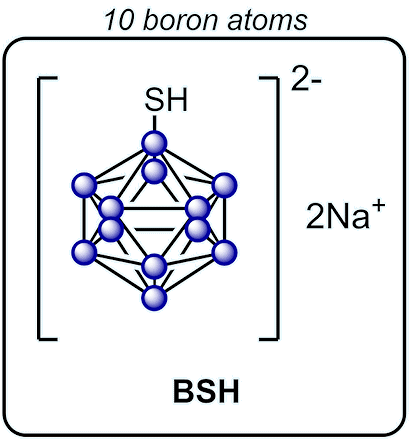 |
 |

Sodium borocaptate (BSH) is the other clinically used BNCT agent alongside BPA. Compared to boronophenylalanine, it carries 10× more boron per molecule.
[Image: self-drawn] |
|
|
On top of that, researchers are testing all sorts of new boron-delivery agents, including antibodies and nanoparticles, to find the most effective one.
Okay, BPA works, but how do we make it?
Chemists have two main tricks for making BPA. One is to start from a natural amino acid and attach a boronic group (-B(OH)2) to it. The other is to start from scratch: attach the boronic group to a benzene derivative and build the amino acid afterwards.
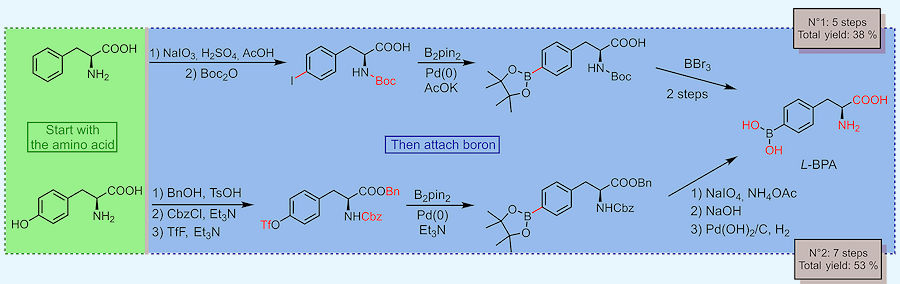
Two synthetic routes to BPA from natural amino acids.
[Scheme: based on Ahmad et al. (2025)]
The first approach seems intuitive, as it takes advantage of naturally occurring amino acids. In these syntheses, chemists start with commercially available, enantiomerically pure L-tyrosine or L-phenylalanine and then attach a boronic group to it. This way the problem with chirality at the alpha carbon is avoided and the only enantiomer produced is the wanted one, L-BPA.
The other strategy is more synthetic. First, the benzene derivative (e.g. 4-bromotoluene in the scheme) is coupled to a boronic group. Then, the amino acid part of the molecule is built. Unfortunately, the end-product is racemic which means it’s an equal mix of the desired L-BPA and the unwanted D-BPA.
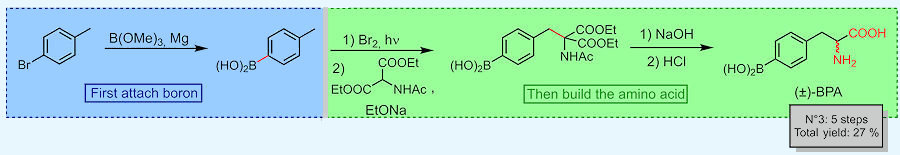
Example of BPA synthesis from 4-bromotoluene.
[Scheme: based on Ahmad et al. (2025)]
All these synthetic pathways leave us with an important question. Which approach seems better and more economical? And why?
Obviously – the shorter, high-yield, amino acid-based synthesis is always better – right?
Yes, that is the case here, but not solely because the synthesis has fewer steps and a higher total yield.
As organic chemists, we usually look at the total yield and the length of the synthesis when comparing different synthetic routes. We especially love short syntheses with high overall yields. In certain cases, however, we might mistakenly consider a synthetic approach to be the best option by focusing only on these two parameters. This is often the case when isotope-enriched materials are involved.
Where’s the catch?
When you’re working with isotope-enriched boron, for instance, an enriched reagent is invaluable compared to other “ordinary” reagents. Every wasted step after it’s added is like pouring money straight down the drain. Hence, chemists design syntheses where the isotope-enriched reagent comes in as late as possible and where the yield of every following reaction is excellent.
This way, a synthesis with more steps and a lower overall yield can actually be superior. What ultimately matters are the length and yield after the incorporation of isotope-enriched elements.
In our case though, there is no doubt. Using amino acids is superior both in the chemical and monetary sense.
Conclusion
BPA, as an unnatural amino acid, is a fascinating example of how chemistry, physics and biology can work together to reveal new horizons in medicine. Although BNCT has not yet become widespread due to challenges with boron delivery and neutron sources, the future of the therapy seems promising. Thanks to BPA, the fight against cancer might have just got a little bit easier.
From lab bench to battlefield – BPA delivers the alpha strike!
[Image: self-drawn]

Bibliography
- Narancic, T., Almahboub, S. A., O’Connor, K. E. (2019). Unnatural amino acids: Production and biotechnological potential. World Journal of Microbiology and Biotechnology, 35, 67. https://doi.org/10.1007/s11274-019-2642-9
- Wikipedia. (27.9.2025). Bisphenol A.
- Wikipedia. (27.9.2025). Amino acid.
- Wikipedia. (27.9.2025). Neutron capture therapy of cancer.
- Barth, R. F., Gupta, N., Kawabata, S. (2024). Evaluation of sodium borocaptate (BSH) and boronophenylalanine (BPA) as boron delivery agents for neutron capture therapy (NCT) of cancer: An update and a guide for the future clinical evaluation of new boron delivery agents for NCT. Cancer Communications, 44(8), 889–892. https://doi.org/10.1002/cac2.12582
- Ahmad, S., Pan, M., Hayward, J. J., Sementilli, M., Porter, L. A., Trant, J. F. (2025). Boron in my mind: A comprehensive review of the evolution of the diverse syntheses of 4-borono-L-phenylalanine, the leading agent for boron neutron capture therapy. ChemMedChem, 20(11), e202500059. https://doi.org/10.1002/cmdc.202500059
- Wongthai, P., Hagiwara, K., Miyoshi, Y., Wiriyasermkul, P., Wei, L., Ohgaki, R., Kato, I., Hamase, K., Nagamori, S., Kanai, Y. (2015). Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2. Cancer Science, 106(3), 279–286. https://doi.org/10.1111/cas.12602
- Hu, K., Yang, Z., Zhang, L., Xie, L., Wang, L., Xu, H., Josephson, L., Liang, S. H., Zhang, M.-R. (2020). Boron agents for neutron capture therapy. Coordination Chemistry Reviews, 405, 213139. https://doi.org/10.1016/j.ccr.2019.213139
- Pizzorno, L. (2015). Nothing boring about boron. Integrative Medicine (Encinitas), 14(4), 35–48. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4712861.


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.30347461]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.30347461]
![]()
![]()
![]()
![]()