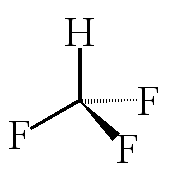
![]()
Fluoroform, CHF3
It's not the same as chloroform!
![]()
Simon Cotton
University of Birmingham, UK
![]()
Molecule of the Month January 2013
Also available: JSMol version.
![]()
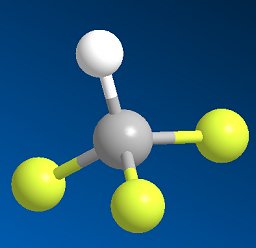
 |
Fluoroform, CHF3It's not the same as chloroform!
Simon Cotton
Molecule of the Month January 2013
|
 |
Exactly, simply replace Cl with F. In fact it is similar to the other CHX3 molecules (X = halogen), the melting and boiling points just increase as the molecules get heavier, with more electrons, and Van der Waals' forces become stronger.
| CHF3 | CHCl3 | CHBr3 | CHI3 | |
|---|---|---|---|---|
| Appearance (298 K) | Colourless gas | Colourless liquid | v. pale yellow liquid | v. pale yellow liquid |
| m.p. (°C) | -163 | -63.2 | 9 | 119.2 |
| b.p. ( °C) | -82.1 | 61.3 | 150-3-151.2 | - |
 So it is an anaesthetic too?
So it is an anaesthetic too?Not that I know of, it is mainly used in the semiconductor industry, for plasma etching of silicon dioxide or silicon nitride. It has been used to put out fires because it is unreactive, not toxic and, for a gas, has a high density. It is also used in refrigerating systems, not least in car air-conditioning.
Quite so, but there are problems with it.
No, it is far too stable for that - and there are no Cl atoms to release. It's the stability that is its downfall, as it has a lifetime in the atmosphere of around 270 years and is a greenhouse gas (HFC-23) with 11700 times the power of CO2. Add to that the fact that it is produced industrially in large quantities as a by-product in the manufacture of chemicals like Teflon, and there's a problem, not least because it's hard to decompose.
Because it is so unreactive, chemists have found it hard to create uses for it, but lately some more possibilities have opened up. A lot of modern medicinal molecules contain fluorine atoms or even CF3 groups, notably Prozac.
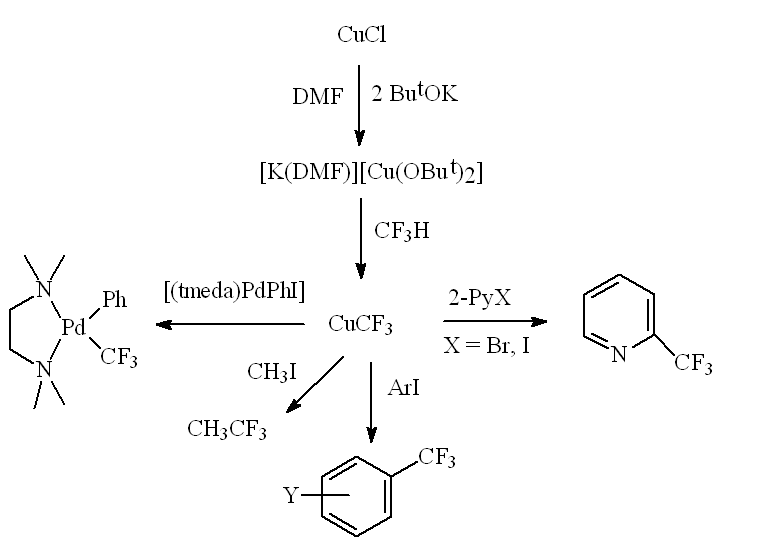
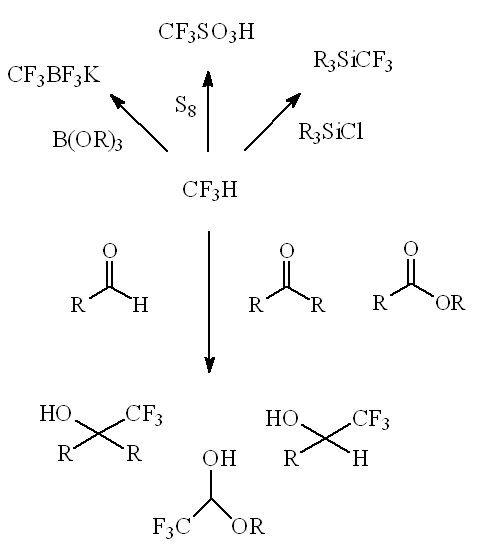
Anionic alkoxycuprates M[Cu(OBut)2] (M = Na, K) react with CHF3 forming CuCF3 derivatives, which efficiently trifluoromethylate a whole range of electrophiles (both organic and inorganic).
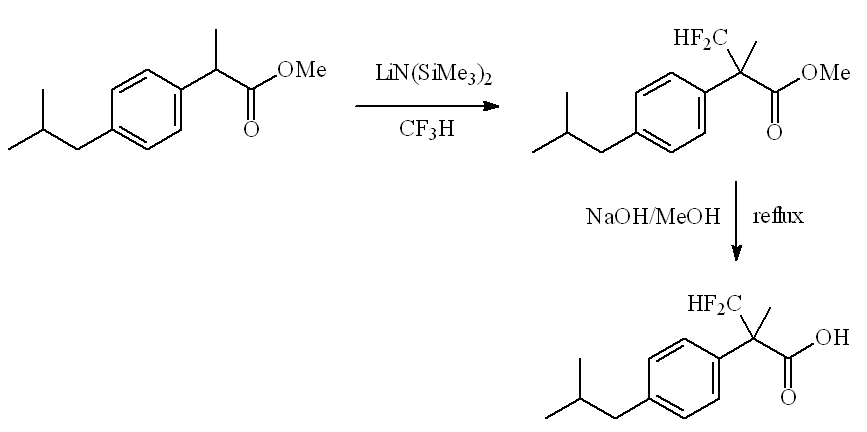
Using an umpolung (reversed polarity) form of CHF3 as a difluoromethyl carbocation equivalent (HCF2+ ion) has enabled difluoromethylation of lithium enolates, as in this synthesis of the α-difluoromethyl analogue of ibuprofen.
Most recently, use of a particular base, KN(SiMe3)2, in a particular solvent (THF) has provided a way to trifluoromethylate a whole range of substances, from silyl chlorides to aldehydes and chalcones, whilst sulfur can readily be converted into the important strong acid CF3SO3H ("triflic acid").
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5258578]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5258578]