![]()
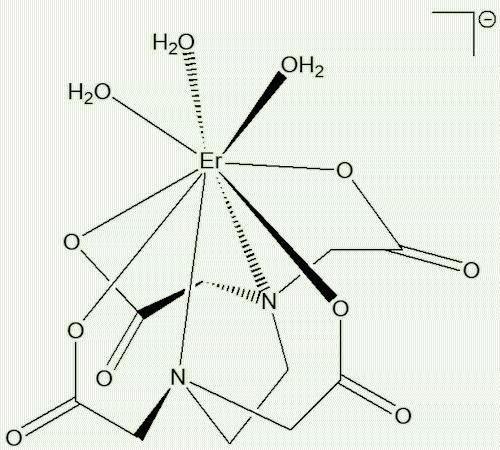
Triaqua(ethylenediamine-
tetraacetato)erbate(III)(1-)
[Er(EDTA)(H2O)3]-
A metal complex with 9 metal bonds
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month October 2024
![]()
|
Triaqua(ethylenediamine-
|
 |
How do you mean?
Transition metals, yes, most of the time. So Co3+ is six coordinate in its complex with the same ligand, EDTA.

Cobalt is a relatively small transition metal ion; the lanthanides, like erbium, are bigger. The comparable ionic radii for Co3+ and Er3+ ions are 0.685 and 1.030 Å, respectively.
A ligand like ethylenediaminetetracetate (EDTA4-), the one used here, can wrap completely around the cobalt(III) ion, leaving no space for any other molecules to bind to it. EDTA cannot do that with the bigger erbium, there is space for up to three water molecules to attach themselves to the metal too. So erbium is nine-coordinate in this complex. These high coordination numbers for lanthanides were only fully established in the 1960s, with Lynn Hoard’s determination of the structure of the nine-coordinate [La(EDTA)(H2O)3]- ion being a breakthrough moment.
By no means, erbium complexes are known with coordination numbers from three to twelve. In fact, coordination number 8 is the most common in lanthanide complexes, followed by 9 and 10; even coordination number 6 is quite uncommon for this metal.
Is erbium typical of the lanthanides?In 1926, Victor Moritz Goldschmidt (1888-1947), a Norwegian mineralogist of Swiss-Jewish descent, published research showing that the lanthanide ions got smaller as their atomic number increased. This is generally referred to as 'the lanthanide contraction'. It affects the heavier transition metals, so that the 5d metals resemble the 4d metals more than might be expected, and this is how Goldschmidt coined the term. So sometimes the early lanthanides have bigger coordination numbers than the later lanthanides in similar compounds. Did Goldschmidt make any other big discoveries?He's regarded as the pioneer of chemical mineralogy, in which area he devised the Goldschmidt classification of the elements. Most of his discoveries were in mineralogy. He was a professor at Göttingen when the Nazis came to power, and Jews started to be threatened, so he left Germany for Norway in 1935. After the Germans invaded Norway, he was arrested by the Nazis, but managed to escape, and eventually reached Britain. He died relatively young. |
 Victor Moritz Goldschmidt [Image: Asta Nørregaard, Public domain, via Wikimedia Commons] |
The early lanthanides generally form M[Ln(EDTA)(H2O)3] and the smaller, later ones, form M[Ln(EDTA)(H2O)2] (where M is a metal ion with a +1 charge like Na or NH4).
For the middle-sized lanthanides, there’s an equilibrium in solution
[Ln(EDTA)(H2O)3]- ![]() [Ln(EDTA)(H2O)2]- + H2O
[Ln(EDTA)(H2O)2]- + H2O
More than that, the stoichiometry of the complex isolated from solution depends to some extent upon the counter-ion, M+. So with holmium, to take one example, Cs[Ho(edta)(H2O)2]·3H2O has eight-coordinate holmium, whilst K[Ho(edta)(H2O)3]·2H2O has nine-coordinate holmium. Similarly with erbium and some other metals.
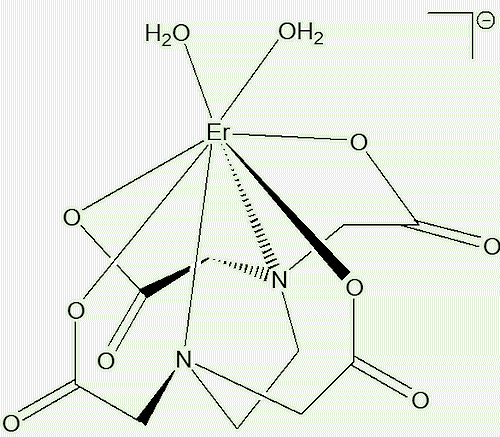
In 2014, two Polish chemists reported studying two EDTA complexes of Er3+. Crystals of [C(NH2)3]2 [Er(EDTA)(H2O)2] ClO4.6H2O contains eight-coordinate Er3+, whilst Na[Er(EDTA)(H2O)3].5H2O contains nine-coordinate Er3+. They examined their electronic spectra, in both the crystal and solution.
Usually the electronic spectra of a particular lanthanide ion do not vary much from one compound to another. But Er3+, along with Nd3+ and Ho3+, is unusual, in that their spectra contain some electronic transitions that are ‘hypersensitive’ to the environment of the metal ion, with the intensities of certain bands changing position, as well as splitting, together with their intensities varying. In the case of these two erbium compounds, the positions of the absorption bands associated with the 4I15/2  4S3/2 hypersensitive transition in the two complexes are rather different. So you can see that in aqueous solution the Er3+-EDTA complex present is predominantly the eight-coordinate [Er(EDTA)(H2O)2]- ion. The equilibrium constant (Kaqua) for the process below is 19 ± 1.
4S3/2 hypersensitive transition in the two complexes are rather different. So you can see that in aqueous solution the Er3+-EDTA complex present is predominantly the eight-coordinate [Er(EDTA)(H2O)2]- ion. The equilibrium constant (Kaqua) for the process below is 19 ± 1.
[Er(EDTA)(H2O)3]- ![]() [Er(EDTA)(H2O)2]- + H2O
[Er(EDTA)(H2O)2]- + H2O
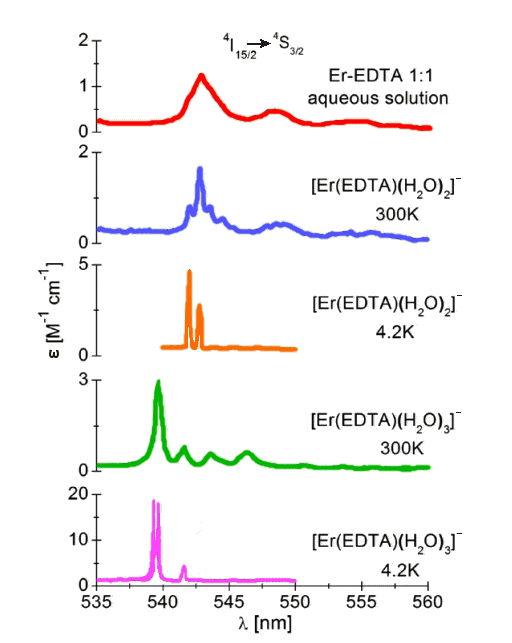
Absorption spectra of the 4I15/2  4S3/2 transition for the [Er(EDTA)(H2O)2]- and [Er(EDTA)(H2O)3]- ions in the solid state as well as the Er3+–EDTA complex in solution.
4S3/2 transition for the [Er(EDTA)(H2O)2]- and [Er(EDTA)(H2O)3]- ions in the solid state as well as the Er3+–EDTA complex in solution.
[Redrawn from data in: R. Janicki and A. Mondry, Phys. Chem. Chem. Phys., 16 (2014) 26823]
Because the lanthanide (III) ions are so similar in their size, they have similar properties, including solubility of their compounds. In their ores they occur as mixtures, so they are difficult to separate. Chemists had to do repeated fractional crystallization, using slight solubility differences between the salts of neighbouring lanthanides, such as the bromates Ln(BrO3)3.9H2O, to achieve this. Famously, 15,000 recrystallizations were carried out by the British-born American Charles James in 1911 to get pure thulium bromate! During the Manhattan Project in World War II, ways had to be found to achieve separations of the radioactive actinide ions, and the use of ion-exchange resins came into being. The smaller the lanthanide, the stronger the complex it forms with EDTA4-, and this ligand was found to give good separations with the heaviest (and smallest) lanthanides washed off the ion-exchange resins first, as shown in the diagram.
|
A long column is filled with a cation-exchange resin, and a small amount of aqueous solution of the mixture of the cations is loaded onto the top of the column. The Ln3+ ions are adsorbed by the resin there. Then the solution of EDTA4- (the ‘eluant’) is added at the top. It dissolves Ln3+ ions from there and then slowly passes over the adsorbed cations a little lower down. The eluent continually exchanges Ln3+ ions with those on the resin. What happens is that the EDTA preferentially complexes the smaller, heavier, metal ions, which form the strongest complexes, so they get concentrated in the solution, whilst the larger metal ions, which form weaker EDTA complexes are ‘left behind’ on the resin. In the diagram, you can see blue and brown ions randomly mixed at the start, but by the time the eluent leaves the column, the blue and brown ions are completely separated. The bluer ions are the smaller of the two metal ions. |
Although they are very similar chemically, each lanthanide has its own unique spectroscopic and magnetic properties. Erbium ions are doped into glass fibres which are employed in fibre amplifiers and lasers. That is erbium’s ‘niche’.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.25350985]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.25350985]