
![]()
Hydrogen Sulphide, H2S
The smell of rotten eggs
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Molecule of the Month March 2009
Also available: HTML version.
![]()
 |
Hydrogen Sulphide, H2SThe smell of rotten eggs
Simon Cotton
Molecule of the Month March 2009
|
Yes, it is formed when proteins contained in the egg are decomposed by bacteria, making H2S, as in the famous Punch cartoon (below), which gave rise to the expression "a curate's egg". Originally the expression meant to call something bad, good, out of politeness or deference; nowadays, the term is used to mean something partly good and partly bad.
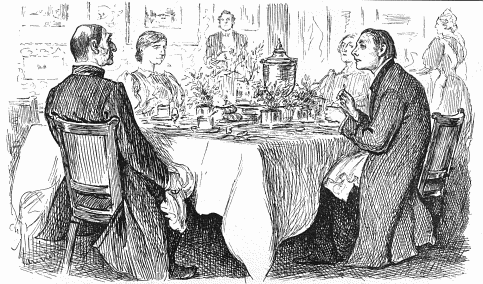
Right Reverend Host: I'm afraid you've got a bad egg, Mr. Jones!
The Curate: Oh no, My Lord, I assure you! Parts of it are excellent!
Drawn by George du Maurier and published in the Nov 9, 1895 issue of Punch
Bacteria in the colon can form H2S there, and it is released as a principal component of flatus. Due to bacteria in the mouth, hydrogen sulphide may also be a cause of bad breath.
 Is that the only source of hydrogen sulphide?
Is that the only source of hydrogen sulphide?Most certainly not, eggs are only a very minor one. Some natural gas fields (such as Lacq in Aquitaine, France (shown right); Canada; China; the USA) yield gas which is often found to contain significant amounts of hydrogen sulphide, and is termed "sour gas".
The H2S can be removed by being converted to sulphur in the Claus process; this is an important industrial source of sulphur. Organic sulphur compounds present in crude oil have to be removed; the sulphur they contain is converted to hydrogen sulphide, or to sulphur itself, which can be stored in blocks (shown below in blocks 25 feet tall on the prairie at Alberta, Canada).
The hydrogen sulphide content can present a real health hazard. There was an accidental sour-gas blow-out from a well in Kaixian County of southwest China at 10 p.m. on December 23, 2003, releasing hydrogen sulphide. 64,000 residents were evacuated, but 243 people died from breathing in the fumes, China's worst gas-field disaster.
 Volcanic gases
Volcanic gasesVolcanoes emit sulphur mainly in the form of sulphur dioxide, but some hydrogen sulphide is released too; this is why the smell of volcanoes is mainly that of SO2 ("burning match") rather than bad eggs. These two gases react together in a redox reaction, forming sulphur, which can often be seen as a yellow deposit round the crater of a volcano (see photo, right).
2 H2S (g) + SO2 (g) ![]() 3S (s) + 2 H2O (g)
3S (s) + 2 H2O (g)
 Surprisingly toxic, roughly comparable to hydrogen cyanide. It can be detected by its "bad egg" smell at levels around 0.005 ppm (parts per million). It can cause eye pain at 5-10 ppm. Levels around 150-250 ppm cause the sense of smell to disappear, and slightly higher levels may well be fatal. At 500 ppm, inhalation can cause immediate collapse and unconsciousness, whilst one breath at the 1000 ppm level results in immediate loss of consciousness, cardiac arrest and death. Taken together, this means that a person exposed to high concentrations may get no warning.
Surprisingly toxic, roughly comparable to hydrogen cyanide. It can be detected by its "bad egg" smell at levels around 0.005 ppm (parts per million). It can cause eye pain at 5-10 ppm. Levels around 150-250 ppm cause the sense of smell to disappear, and slightly higher levels may well be fatal. At 500 ppm, inhalation can cause immediate collapse and unconsciousness, whilst one breath at the 1000 ppm level results in immediate loss of consciousness, cardiac arrest and death. Taken together, this means that a person exposed to high concentrations may get no warning.
Inhibition of cytochrome c oxidase in a similar manner to HCN has been suggested as a reason for its toxicity, but others believe that it could also involve a reactive S-containing species which depletes glutathione and activates oxygen.
Apart from people working with "sour gas" fields of natural gas, sewage workers are particularly at risk, as stagnant sewage can release large amounts of H2S when agitated, and fatalities are regularly reported. H2S is denser than air, and can accumulate in the bottom of unventilated spaces, such as sewers. The celebrated Bogle-Chandler case - the mysterious deaths of Dr Gilbert Bogle and Mrs Margaret Chandler on a Sydney riverbank in 1963 - has recently been attributed to H2S released from the Lane Cove River.


Left: Warning sign to wear a gas-mask in enclosed spaces if H2S is present.
Right: Warning sign from Germany, which says "No ice-skating - danger of life - hydrogen sulphide in the water".
It's been suggested that H2S was responsible for a huge mass extinction of life about 250 million years ago, the "Permian-Triassic extinction event". The eruption of Siberian volcanoes led to large amounts of volcanic CO2, leading to warming that depleted oceanic oxygen levels. This in turn led to oceanic bacteria reducing sulphate to produce large amounts of H2S, killing aqueous plants and animals, both in the oceans and on land.
Yes, but there may just be medical uses for H2S. Very low doses of it can reduce metabolic rate, and it has been suggested that this could allow organ function to continue even when oxygen supply is limited, after a traumatic injury, for example. This suspended-animation state may be suitable for surgical applications. It has been suggested that healthy properties of garlic may be due to H2S; when crushed garlic is added to red blood cells H2S is released, and it is known to act as a vasodilator, opening blood vessels. Hydrogen sulphide has been found to inhibit formation of the damaging O2- ion (superoxide) in muscle tissue.
As it's so toxic, it is not a good idea for an inexperienced chemist to try to make it. In the laboratory, it is traditionally made from the reaction of lumps of iron(II) sulphide with dilute acid (e.g. HCl):
FeS (s) + 2 H+ (aq) ![]() Fe2+ (aq) + H2S (g)
Fe2+ (aq) + H2S (g)
Generations of chemistry students were familiar with the Kipp's apparatus, named after the Dutch pharmacist Petrus Johannes Kipp (1808-1864). It can be used to generate small quantities of a gas (not just H2S) "on demand". To make H2S, pieces of FeS are placed in the middle vessel, and acid into the top vessel. When the tap attached to the middle vessel is opened, acid runs down into the bottom vessel and then up into the middle one, reacting with the FeS and generating the gas. Closing the tap causes gas pressure to increase, forcing the acid back down into the bottom vessel and stopping the reaction.
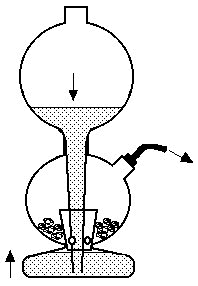

Kipp's apparatus for making H2S
 H2S was widely used in qualitative analysis for years, as it reacts with solutions of many metals forming precipitates (thus Cd2+ forms a characteristic yellow precipitate of CdS). Sodium sulphide and thioacetamide are safer alternatives to H2S gas as a source of sulphide. Pb2+ forms a black precipitate of PbS. This is also formed on paintings in city art galleries, when traces of hydrogen sulphide from gas lighting or coal fires reacted with "white lead" pigment, lead carbonate. The white colour in paintings can be regenerated by treatment with hydrogen peroxide, forming white lead sulphate, PbSO4.
H2S was widely used in qualitative analysis for years, as it reacts with solutions of many metals forming precipitates (thus Cd2+ forms a characteristic yellow precipitate of CdS). Sodium sulphide and thioacetamide are safer alternatives to H2S gas as a source of sulphide. Pb2+ forms a black precipitate of PbS. This is also formed on paintings in city art galleries, when traces of hydrogen sulphide from gas lighting or coal fires reacted with "white lead" pigment, lead carbonate. The white colour in paintings can be regenerated by treatment with hydrogen peroxide, forming white lead sulphate, PbSO4.
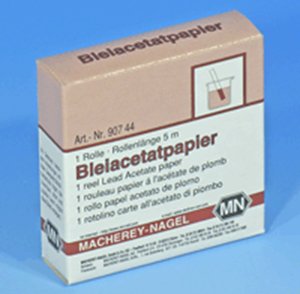
The reaction with lead salts is the basis for the traditional test for hydrogen sulphide gas. Moist lead acetate (lead ethanoate) paper (see image, right) turns from white to silvery grey or black when exposed to H2S.
Pb2+ (aq) + H2S (g) ![]() PbS (s) + 2 H+ (aq)
PbS (s) + 2 H+ (aq)
Another type of hydrogen sulphide test is used to test for its presence in water, particularly to show the absence of faecal coliform bacteria in the sample, meaning that it is safe to drink. Bacteria of the Enterobacteriacae group will reduce cysteine (and certain other sulphur compounds, as well as S itself) forming H2S. This test uses thiosulphate to provide the sulphur, the enteric bacteria reducing it to H2S; and ferric ammonium citrate to indicate the presence of H2S, by forming a black precipitate of FeS. A suitable adsorbent paper is soaked in a solution made from bacteriological peptone, dipotassium hydrogen phosphate, ferric ammonium citrate, sodium thiosulphate; it is exposed to the water sample for 3 days, whereupon a black colour (and a smell of H2S) would demonstrate the presence of hydrogen sulphide-producing organisms. This test has been recommended for use in small communities which do not have conventional water treatment systems nor the infrastructure to monitor water quality, such as many Pacific Islands.
 Silver cutlery exposed to town air (which contains traces of H2S) form a black tarnish of Ag2S, removed by polishing. A similar tarnish is formed if eggs are eaten with silver spoons.
Silver cutlery exposed to town air (which contains traces of H2S) form a black tarnish of Ag2S, removed by polishing. A similar tarnish is formed if eggs are eaten with silver spoons.
Bismuth subsalicylate sharply reduces colonic H2S release, probably because of the extreme insolubility of Bi2S3 (Ksp = 1.8![]() 10-99).
10-99).
The H2S molecule has a similar V-shape. Because sulphur is a bigger atom than oxygen, the H-S bonds are longer than the H-O bonds in water.

The dipole moment of hydrogen sulphide is 0.97 Debye, less than that of H2O (1.84 D), meaning that it is a less polar molecule. This is due to the smaller electronegativity difference between H and S making the H-S bonds less polar than O-H. Another consequence of the low polarity of the S-H bonds is that H2S molecules do not associate by hydrogen-bonding, as water molecules do. This means that H2S has a much lower boiling point, -60.3°C rather than 100°C. Unlike ice, which contains an open lattice of water molecules hydrogen-bonded to each other, solid hydrogen sulphide contains H2S molecules in close packing.
What determines the shape of a molecule is the repulsion between the electron pairs round the central atom; they try to get as far away from each other as they can. Like water, H2S has four central electron pairs, arranged approximately tetrahedrally, so do the analogous H2Se and H2Te. The two non-bonded pairs produce stronger repulsions than the pairs in the M-H bonds, so the two bond pairs are squeezed together, and the H-M-H angle is below 109½°.
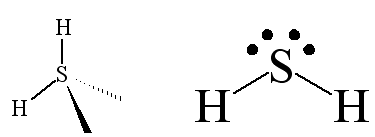
Looking down the series of H2M, as the central atom becomes less electronegative, the bond pairs of electrons move more towards hydrogen, the reduced repulsion between the bond pairs allows the H-M-H bond angles to close up. An alternative view of the bonding in these compounds is that in H2M (M = S, Se, Te) the H-M bonds are mainly derived from interaction of the 1s orbital of hydrogen with the outermost p orbital of M, with little contribution from its s orbital, whereas in H2O the oxygen atom participates in hybridisation to relieve the greater repulsion between the two O-H electron clouds.
Other properties in H2M follow patterns, with water usually being exceptional. The anomalously high boiling point for H2O is due to intermolecular hydrogen bonds; for the others, the Van der Waals' intermolecular forces get stronger as the molecular size (and numbers of electrons) increases, causing the melting and boiling points to increase as M gets heavier.
| H2O | H2S | H2Se | H2Te | |
| M.P. / °C | 0 | -82.9 | -64.0 | -48.9 |
| B.P. /°C | 100.0 | -59.4 | -42.0 | -2.2 |
| Density of liquid / (g cm-3) | 1.00 | 0.993 (-85.6°C) | 2.12 | 2.68 |
| Density of solid /(g cm-3) | 0.917 | 1.12 (-85.6°C) | ||
| ΔfH /(kJ mol-1) | -286 | 20.1 | 73.0 | 99.6 |
| M-H bond length / pm | 95.7 | 133.6 | 146 | 169 |
| Angle H-M-H /° | 104.5 | 92.1 | 91 | 89.5 |
![]()
![]()