
POLYVINYL CHLORIDE
(a.k.a. PVC, poly(chloroethene), ‘vinyl’)
Records, tubing, flooring and unusual clothing...

Simon Cotton
University of Birmingham

Molecule of the Month June 2017
Also available: HTML version.

|

Geri Halliwell (Ginger Spice) almost wearing a PVC dress. |
 Where does ‘vinyl’ originate?
Where does ‘vinyl’ originate?
You have really asked two different questions. When most people say ‘vinyl’, they may mean a type of gramophone record, the flooring material, which is also used to make water pipes and window frames, or material used to make clothing (though that is usually referred to as PVC).
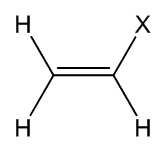
For chemists, vinyl traditionally means the H2C=CH- grouping in a molecule (just as ethyl means CH3CH2-). This name was coined by the celebrated German chemist Hermann Kolbe (photo, right) in 1851.
 |
 |
|
| The vinyl group |
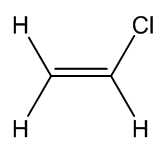
Chloroethene
a.k.a. vinyl chloride |
|
So it is one of those modern materials?
Actually it was first made by accident in the 19th century - just like several other polymers, including poly(ethene) and poly(styrene). It was first reported in 1835, and rediscovered in 1872. When gaseous vinyl chloride (b.p. –13.4°C) was exposed to sunlight for a few days, a white solid formed. This was PVC. It was patented in 1913.
How do you make it?
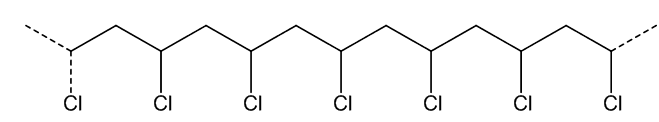
It is made by polymerising chlorethene (vinyl chloride), analogously to turning ethene into poly(ethene) (MOTM December 2006) or tetrafluorethene into PTFE (MOTM June 2009), using a catalyst such as dioctanoyl peroxide. This splits at the peroxide link, generating the radicals that catalyse the reaction (MOTM benzoyl peroxide).
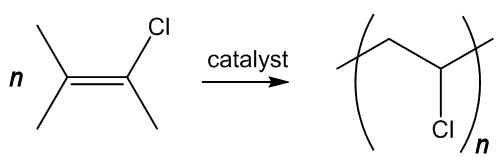
As the double-bond is lost, the geometry at the carbon atoms changes. Like ethene or tetrafluoroethene, chloroethene is a flat molecule, with sp2 hybridisation of carbon atoms and bond angles of approximately 120°. On polymerisation, as each carbon is bound to four separate atoms, hybridisation changes to sp3, and the bond angle at carbon to approximately 109.5°.
And this is the stuff we’re familiar with today?
Well, normally they add a plasticiser to the PVC.
I thought that PVC was already a plastic?
There’s a problem, we use the words ‘polymer’ and ‘plastic’ as it they are interchangeable, and they are not. PVC is a giant molecule, a polymer of vinyl chloride, but it isn’t always plastic. A substance is described as ‘plastic’ if it is a material that is capable of being moulded or shaped. A plasticiser is a substance that adds to the flexibility of a polymer, making it easier to shape.
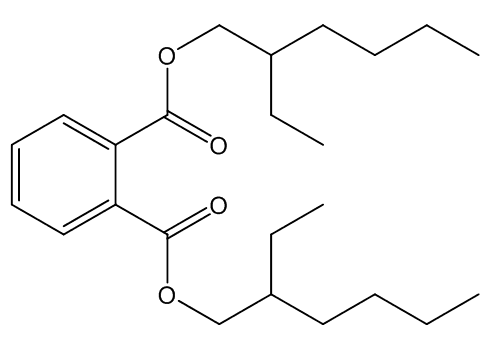
The most widely used plasticisers are phthalates, one of the commonest of those is bis(2-ethylhexyl)phthalate:
 |
|
| Bis(2-ethylhexyl)phthalate |
|
The downside of plasticisers is that they can leach out, and some are prohibited from use in toys for children, especially toys that may be put into the mouth; they appear to affect hormone levels and there are possible links with breast cancer in women.
So when did PVC come into wide use?
The original PVC was too brittle to be much use. The key discovery was someone looking for an artificial rubber material that would stick to metals. That someone was Waldo Lonsbury Semon (1898-1999), and he was working for the American B.F. Goodrich Company. He tried mixing PVC with various solvents and sometimes got gels that could be moulded. None of the applications they tried seemed commercially important, but then Semon made a breakthrough.
What was that?
Semon’s wife had been making new curtains for the living room, and so, as he went on to say: "I brought some of the fabric into the laboratory and coated it with PVC, and lo and behold, it looked like silk and it was waterproof... I became so enthusiastic... I forgot about protocol and went directly to the vice president of sales, and he looked at it and he says, 'Hell, what do you mean, waterproof?' So I grabbed the fabric and put it on top of his incoming mail and took a decanter of water and poured it. Oh, he was really frightened, but it didn't leak... I've often wondered what would have happened to me or to PVC if it had leaked."
He got a patent in 1933 for plasticised PVC. It became the hot (and waterproof!) material for shower curtains, umbrellas and raincoats.
And other applications?
Yes, one of the best known is ‘vinyl’ records. These usually employ a copolymer with vinyl acetate (PVC/PVA).
 |
 |
 |
| Vinyl records |
PVC-PVA co-polymer |
Credit cards |
They replaced shellac as the material of choice for making ‘gramophone’ records just after World War II and were used in huge quantities until CDs (made from polycarbonate) started to be the medium of choice from the early 1980s (the first was ABBA’s The Visitors). Now vinyl records are fashionable again, both collecting old records and new releases.
Credit cards are also usually made of a PVC/PVA copolymer, though there are other materials used in them, like the iron oxide for the magnetic strip, and the chip.
 |
 |
 |
| PVC flooring |
PVC pipes and guttering |
uPVC double-glazing |
PVC is chemically inert, a non-conductor of electricity as well as fire-resistant. These properties account for its myriad uses, including flooring and electrical insulation for wiring and cables, as well as in medical circles, as the material for making both tubing and vessels to contain blood and other substances. Its weather resistance explains the use of unplasticised PVC (uPVC) in window-frames and doors, and PVC is commonly used these days to make the piping to carry both water and sewage (not at the same time, obviously); unlike metal pipes it does not corrode. PVC is still controversial, though.
How?
Well, PVC clothing is something of a fashion statement, with overtones of kinkiness, but the really controversial thing is toxicity.
 I thought that plastics were inert.
I thought that plastics were inert.
Vinyl chloride by itself is toxic, which was only discovered in the 1970s, when people working in PVC manufacturing were found to have liver and spleen damage. PVC is much safer, but it is claimed that if you burn PVC in an incinerator you get toxic dioxins (MOTM September 2005), but improving incinerator conditions, notably by working at high temperatures, breaks down these unwelcome pollutants. Feedstock recycling is also a means of minimising emissions.
Bibliography
General
- Chapman and Hall Combined Chemical Dictionary code number: CXM64-C (monomer).
- Polymers: A Properties Database (part of the CRC Press CHEMnetBASE): Poly(vinyl chloride) unplasticised, reference number 490
- H. Kolbe, J. Chem. Soc., 1851, 3, 369-411 (origin of the name vinyl)
- S. Fenichell, Plastic: The Making of a Synthetic Century, New York, Harper Collins, 1997, passim.
- R. L. Myers, The 100 Most Important Chemical Compounds. A Reference Guide. Westport, Connecticut, Greenwood Press, 2007, pp 295-297.
- H. V. Regnault, Ann. Chim. Phys., 1835, 58, 301-335. (discovery)
- E. Baumann, Annalen der Chemie und Pharmacie, 1872, 163, 308–322. (discovery) "Über einige Vinylverbindungen" (On some vinyl compounds), DOI: 10.1002/jlac.18721630303
- German Patent 281 877 Feb. 1915. (PVC as a substitute for horn and for films, fibres, and lacquers)
- W. Semon, US Patent 1,929,453 (Patented Oct 10. 1933) ‘Synthetic Rubber-like Composition and Method of Making Same’.
- W. Semon, US Patent 2,188,396 (January 30 1940) ‘Method of preparing polyvinyl halide products’
- Obituary of Walso Lonsbery Seman)
- M. W. Allsopp and G. Vianello, Ullmann's Encyclopedia of Industrial Chemistry, 2000, Vol 28, pp 441-468.
- I. Fischer, W. F. Schmitt, H. C. Porth, M. W. Allsopp and G. Vianello, Ullmann's Encyclopedia of Industrial Chemistry, 2014, Vol 28, pp 441 468.
- D. Braun, J. Vinyl & Additive Technol., 2001, 7, 168–176 (PVC - Origin, Growth, and Future)
- D. Braun, Kunststoffe International, 2010, pp 30-33. (PVC: A Many Faceted Polymer)
- Claudia Flavell-While, The Chemical Engineer, May 2010, pp 52-53 (Semon’s discovery of plasticised PVC)
- PVC Centenary (100 years of PVC)
Credit cards
Vinyl records
PVC combustion and dioxins
- R.Weber et al., Environ. Sci. Pollut. Res., 2008, 15, 363–393 (dioxin- and persistent organic pollutant-contaminated sites—contemporary and future relevance and challenges)
- E. Weidemann, S. Marklund, H. Bristav and L. Lundin, Chemosphere, 2014, 102, 12-17 (in-filter PCDF and PCDD formation at low temperature combustion)
- H. Zhou, A. Meng, Y. Long, Q. Li and Y. Zhang, Waste Management, 2015, 36, 106-118. (review: dioxin-related substances during municipal solid waste incineration)
- E. Weidemann, E. Allegrini, T. Fruergaard Astrup, T. Hulgaard, C. Riber and S. Jansson, Waste Management, 2016, 49, 110–113
- https://waste-management-world.com/a/pvc-to-burn-or-not-to-burn


 Back to Molecule of the Month page.
Back to Molecule of the Month page.
![]()
![]()
![]()
![]()


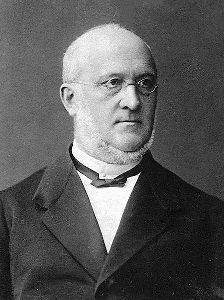 Where does ‘vinyl’ originate?
Where does ‘vinyl’ originate?









 I thought that plastics were inert.
I thought that plastics were inert.