Sounds like a science fiction serial
No, it is probably the most dangerous greenhouse gas.
 I thought that was carbon dioxide?
I thought that was carbon dioxide?
SF6 is the gas with a global warming potential, measured over a 100-year span, around 20,000 times greater than CO2.
So it is a nasty substance?
Not at all, it is very unreactive. It has an atmospheric lifetime of about 3,200 years; it is its lack of reactivity and persistence that magnifies the problem, as it is so slow to break down and disperse.
 Where does it come from?
Where does it come from?
It was first reported in 1900 by Henri Moissan (picture, left), the discoverer of fluorine. It is usually made by burning sulphur in an atmosphere of fluorine, though it can be prepared by pyrolysis of SF5Cl (whose synthesis does not require the use of elemental fluorine).
S + 3F2  SF6
SF6
The SF6 has to be purified rigorously before use, to eliminate reactive fluorides; washing with a KOH spray removes S2F2, SO2F2, and SF4, followed by pyrolysis at 400°C, which converts S2F10 into a mixture of SF4 and SF6. This remaining SF4 is removed with a KOH spray; the SF6 is then dried and passed through charcoal to remove any trace impurities. The purity of the SF6 is checked by exposing albino mice to a mixture of 21% oxygen and 79% SF6 for 24 hours.
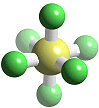
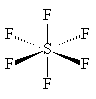
It is a colourless, tasteless, non-flammable, non-toxic and insoluble gas at room temperature. SF6 is made of octahedrally-shaped molecules, with a bond length of 1.561 Å (gas phase); SeF6 and TeF6 also exist, though these are more reactive. Other octahedral fluorides are known, but they all contain a metal, as in UF6 and PtF6. Like other covalent substances, there are strong covalent bonds within the molecules but only weak (Van der Waals') forces between molecules, so that it has a low boiling point and melting point (actually it sublimes, at -63.7°C). Nearly all the hexafluorides are very reactive; SF6 is an exception.
So it really is very unreactive?
SF6 can be heated to 500°C without any decomposition and it does not react with high-pressure steam either. It does not attack glass, even up to its softening temperature; metals like magnesium and copper do not react, even at red heat, and it does not attack molten sodium, though it does react with boiling sodium to form Na2S and NaF. Phosphorus and arsenic distil unchanged in SF6 vapour.
Its unreactivity is probably due to a combination of factors. Because fluorine forms strong covalent bonds, the S-F bonds have high bond energies and are difficult to break. Because there are so many fluorine atoms surrounding the sulphur atom, it is well shielded from attacking molecules too. It is likely that it simply reacts extremely slowly owing to high activation energies for its reactions, as hydrolysis is energetically extremely favourable.
SF6(g) + 6H2O (g)  SO3 (g) + 6HF (g) ΔG
SO3 (g) + 6HF (g) ΔG = -301.2 kJ mol-1
= -301.2 kJ mol-1
In 2000, scientists reported the discovery of an even more potent gas in the stratosphere, SF5CF3. No one knows how it is formed from SF6 - people have tried to make it in corona discharges, unsuccessfully - but it was also detected at a concentration of 0.1 parts per trillion in Antarctic snow.
Why does SF6 get into the atmosphere?
SF6 has found some specialist applications because of its unreactivity and lack of toxicity, and some of it escapes.
What is it used for?
It is an extremely good electrical insulator, much better than air, thus it is widely used in the electric power industry as an insulating gas in electrical transmission and distribution equipment, such as circuit breakers and switchgear. Leaks occur rarely, but they can happen when the stations are damaged or simply old. No alternative is yet available.

 It has also been used in the manufacture, casting and degassing of molten aluminium and magnesium; because it is so unreactive, it forms a dense blanket that keeps atmospheric oxygen and nitrogen away from the hot metals during processes like diecasting (these very reactive metals even react with nitrogen when hot).
It has also been used in the manufacture, casting and degassing of molten aluminium and magnesium; because it is so unreactive, it forms a dense blanket that keeps atmospheric oxygen and nitrogen away from the hot metals during processes like diecasting (these very reactive metals even react with nitrogen when hot).
If air containing SF6 is inhaled, the pitch of the person's voice is lowered, as it is a large, heavy, molecule, which reduces the velocity of sound waves, the converse of the better-known phenomenon with helium. Since the 1970s, SF6 has been used in double-glazed windows to improve sound insulation, due to the reduced velocity of sound. It is, however, no good for thermal insulation.
 A rubber balloon filled with SF6 will expand and ultimately burst, as the smaller and faster-moving O2 and N2 molecules diffuse through the rubber skin into the balloon very much faster than SF6 can diffuse out. SF6 has been used to inflate car tyres, and Nike employed SF6 for air cushioning its Air Max shoes from 1978 onwards, as the use of this large molecule minimised leaks from the air cushions in the heel. Though the greenhouse potential of SF6 was recognised in 1992, it took them nearly 14 years to solve the problem; in the end, Nike used 65 layers of plastic to reduce nitrogen loss, and employed blow moulding to produce the desired product, the SF6-free AirMax 360 (photo, left).
A rubber balloon filled with SF6 will expand and ultimately burst, as the smaller and faster-moving O2 and N2 molecules diffuse through the rubber skin into the balloon very much faster than SF6 can diffuse out. SF6 has been used to inflate car tyres, and Nike employed SF6 for air cushioning its Air Max shoes from 1978 onwards, as the use of this large molecule minimised leaks from the air cushions in the heel. Though the greenhouse potential of SF6 was recognised in 1992, it took them nearly 14 years to solve the problem; in the end, Nike used 65 layers of plastic to reduce nitrogen loss, and employed blow moulding to produce the desired product, the SF6-free AirMax 360 (photo, left).
Most of these uses are now being phased out in Europe.
SF6 concentrations in the ppb and even ppt range can be measured by IR spectroscopy and gas chromatography, and because of this - and also its unreactivity - SF6 has come into fashion as an environmental tracer. Thus, SF6 has been suggested as a tracer for underground water leaks and burst water pipes; similarly, it was used in tests at St John's Wood Underground station in North London on March 25th 2007 when its dispersion was monitored to show how a nerve gas might spread during a terrorist attack.


Bibliography
- H. Moissan and P. Lebeau, Comptes Rendus, 1900, 130, 865, 984; Ann. Chim. Phys., 1902, 26, 145 (synthesis).
- J.E. Macintyre (ed), Dictionary of Inorganic Compounds, Chapman and Hall, London, 1992, entry IC-018671.
- Kirk-Othmer, Encyclopedia of Chemical Technology, 4th edition , 1997, John Wiley & Sons, Vol 11, pp.428-435 (review).
- Ullman's Encyclopedia of Industrial Chemistry, Wiley-VCH, 6th edition, 2003, 14, pp.428-429.
- G.W. Rayner-Canham and A. Hewlin, SF6: The most potent greenhouse gas, Education in Chemistry, 2000 (3), 69-70.
- A.G. Massey, Main Group Chemistry, Ellis Horwood, Chichester, 1990, pp.313, 336-7.
- R.J. Gillespie and I. Hargittai, The VSEPR Model of Molecular Geometry, Allyn and Bacon, Boston, 1991, pp.144-145.
- L.S. Bartell and S. K. Doun, J. Mol. Struct., 1978, 43, 245. (gas-phase electron-diffraction).
- B.M .Powell, M.T. Dove, G.S. Pawley and L.S. Bartell, Mol. Phys., 1987, 62, 1127-1141 (powder diffraction)
- http://www.esc.cam.ac.uk/astaff/dove/publications/MolP_SF6_1987.pdf
Vibrational spectrum
SF5CF3
- W.T. Sturges, T.J. Wallington, M.D. Hurley, K.P. Shine, K. Sihra, A. Engel, D.E. Oram, S.A. Penkett, R. Mulvaney, and C.A.M. Brenninkmeijer, Science, 2000, 289, 611, http://www.abc.net.au/science/news/stories/2000/157654.htm
- W. Carrier, C.S. Jamieson, and R.I. Kaiser, Inorg. Chem., 2007, 46, 1332 (formation in irradiated ice).
- R. Tuckett, "SF5CF3 - a 'super' greenhouse gas", Educ. Chem., (2008) 17-21.
Water leak detection
- http://www.scienco.com/news1a.htm
- The Use of Sulphur Hexafluoride as a Conservative Tracer in Saturated Sandy Media, R.D. Wilson and D.M. Mackay, Ground Water, 1993, 31, 719.
Nike shoes
Controlling emissions


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5249137]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5249137]
![]()
![]()
![]()
![]()
 I thought that was carbon dioxide?
I thought that was carbon dioxide? Where does it come from?
Where does it come from?


