
![]()
Arsine
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Molecule of the Month January 2005
Also available: JSMol version.
![]()
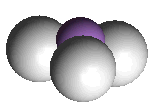
 |
Arsine
Simon Cotton
Molecule of the Month January 2005
|
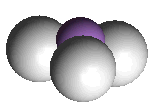 |
No, that's Arsène Wenger.
No, but it is an important molecule in the history of forensic science.
Why?Arsenic was a favourite of early 19th century poisoners. They could obtain it from sources like rat poisons and flypapers. It was hard to detect. Symptoms of arsenic poisoning could be confused with cholera. So it was really easy to get arsenic?Until 1851, when the passing of the Arsenic Act meant that a buyer of arsenic had to be over 21 and known to the chemist. They also had to sign a register. It also stipulated that, to prevent them being confused with sugar or flour, arsenic compounds had to be coloured (with soot or indigo). The Arsenic Act was still only a partial solution, however. How could arsenic be detected?In 1836, an Englishman working at Woolwich Arsenal called James Marsh (1794-1846) developed what became known as Marsh's test for arsenic. It soon came into widespread use, and relied on making arsine, then decomposing it. |
 Film poster from the 1944 movie 'Arsenic and old lace', about a family of poisoners. |
Body remains are reduced by zinc and dilute H2SO4, when any arsenic is turned into gaseous AsH3 (arsine). Obviously the zinc, and other chemicals used, must themselves be free of traces of arsenic. The AsH3 is passed through a heated tube where it is decomposed to arsenic and hydrogen, forming a black mirror film of arsenic in a cold zone.
2 AsH3 ![]() 2 As + 3 H2
2 As + 3 H2
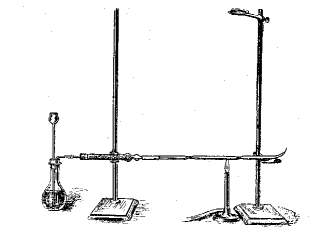
Marsh test apparatus
Diagram from W.A.Campbell, Chemistry in Britain, 1965, 1, 200-201.
 The intensity of the stain can be compared with standard films produced with known amounts of arsenic. The test came to popular attention in the trial in 1840 of the 24-year old Marie Lafarge (left), who was charged with killing her husband Charles; hers was an arranged marriage, and she was unhappy. Her husband had died of a gastric complaint; he had eaten a slice of cake baked by his wife. Their maid swore that she had seen Marie adding a white powder to his drink, and it was shown that she'd twice bought arsenic (oxide) from the local chemist, allegedly to kill the rats at her husband's forge. However, application of the Marsh test to his body showed no arsenic present (though they could detect it in food in the Lafarge house). Mathieu Joseph Bonaventure Orfila, a chemist appearing for the defence, claimed that the test was unreliable. He checked the materials, and repeated the test on Lafarge's body. He found arsenic.
The intensity of the stain can be compared with standard films produced with known amounts of arsenic. The test came to popular attention in the trial in 1840 of the 24-year old Marie Lafarge (left), who was charged with killing her husband Charles; hers was an arranged marriage, and she was unhappy. Her husband had died of a gastric complaint; he had eaten a slice of cake baked by his wife. Their maid swore that she had seen Marie adding a white powder to his drink, and it was shown that she'd twice bought arsenic (oxide) from the local chemist, allegedly to kill the rats at her husband's forge. However, application of the Marsh test to his body showed no arsenic present (though they could detect it in food in the Lafarge house). Mathieu Joseph Bonaventure Orfila, a chemist appearing for the defence, claimed that the test was unreliable. He checked the materials, and repeated the test on Lafarge's body. He found arsenic.
So what happened to Marie Lafarge?Marie was found guilty and sentenced to death, though the sentence was commuted subsequently to life imprisonment. Who was this Orfila guy?Mathieu Joseph Bonaventure Orfila (1787-1853), now known as the "Father of Toxicology," was born in Minorca. He went to study in France and graduated in 1811. In 1819 he was appointed professor of medical jurisprudence, and four years later he succeeded the great L.N.Vauquelin as professor of chemistry in the faculty of medicine at Paris. |
 Mathieu Joseph Bonaventure Orfila |
Within a decade of Marsh's discovery, his test came into regular use, as in the case of 18 year old Catherine Foster, the last woman to be hanged at Bury St Edmunds (Suffolk), in 1847.
In the testimony of E.W.Image…
"I am a surgeon residing in Bury. I remember Mr Jones of Melford bringing me a stomach and some fluid. It was in a jar. It was secured being tied on. Mr S. Newham was there. I proceeded to make an analysis of the contents of the jar that day. Mr Jones stayed to see the analysis… The next test we applied was the Marsh's test by which arsenic was also detected. No doubt was left in our minds..."
And is Marsh's test still used today?Nowadays neutron activation analysis is the most sensitive technique. Arsenic can be detected in nails, hair, and urine in poisoning cases. Famously, high levels of arsenic have been detected in samples of hair said to have been taken from the body of Napoleon Bonaparte, giving rise to suggestions that he was poisoned during his exile on St Helena. And was he?Wallpaper samples from his bedroom show copper arsenite, a green dye used in the 19th century. It has been found that when it is used in damp houses, moulds growing on the paper cause the formation of compounds including trimethylarsine (Me3As) and possibly arsine itself, so one possibility is that he was accidentally poisoned. Others think it was done deliberately. |
 Napoleon Bonaparte |
 And since then?
And since then?Arsenic has regularly cropped up in detective fiction, notably Dorothy L. Sayers' (photo, right) Strong Poison (1930), which features the Marsh test, Agatha Christie's After the Funeral (1953) and Patricia Cornwell's Cause of Death (1996).
It binds to sulphur atoms in enzymes, inhibiting enzyme action.
At room temperature, AsH3 is a highly toxic gas with a garlic-like smell. It can be made by reactions like the reduction of As4O6 with LiAlH4 or AsCl3 with NaBH4. Its thermal instability (decomposed to As and H2 at 250-300°C) is of course used in Marsh's test. Unlike ammonia (but like the other MH3 (M = P, Sb, Bi), AsH3 has a very low basicity but can be protonated by the "Superacid" HF/SbF5 to AsH4+ SbF6-.
There are four electron pairs around arsenic, a lone pair and three bond pairs. The molecule has a trigonal pyramidal shape, similar to ammonia, but with a smaller bond angle.
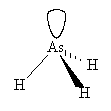 |
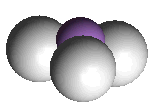 |
| Arsine | and its spacefill representation |
Looking down the series of MH3, as the central atom becomes less electronegative, the bond pairs of electrons move more towards hydrogen, the reduced repulsion between the bond pairs allows the H-M-H bond angles to close up.
Other properties in MH3 follow patterns, with ammonia usually being exceptional. The anomalously high boiling point for NH3 is due to intermolecular hydrogen bonds; for the others, the Van der Waals' intermolecular forces get stronger as the molecular size (and numbers of electrons) increases, causing the melting and boiling points to increase as M gets heavier.
| NH3 | PH3 | AsH3 | SbH3 | BiH3 | |
|---|---|---|---|---|---|
| B.P. (°C) | -34.5 | -87.5 | -62.4 | -18.4 | 16.8 |
| Density of liquid (g/cm3) | 0.683 | 0.746 | 1.64 | 2.20 | - |
| ΔHf (kJ/mol) | -46.1 | -9.6 | 66.4 | 145.1 | 277.8 |
| M-H bond length (pm) | 101.7 | 141.9 | 151.9 | 170.7 | - |
| Angle H-M-H (°) | 107.8 | 93.6 | 91.8 | 91.3 | - |
| Temp of fast decomposition (°C) | - | - | 250 | 20 | -45 |
| Forms salts easily with acids ? | Yes | No | No | No | No |
| M-H bond energy (kJ/mol) | 391 | 322 | 247 | 255 | - |
 What are the symptoms of arsenic poisoning?
What are the symptoms of arsenic poisoning?Arsenic poisoning is accompanied by many symptoms, including a metallic taste in the mouth, diarrhoea, vomiting, garlic-like breath, stomach pains, skin lesions (see photo, right) and various cancers; it tends to affect the kidneys, also the liver, lungs and skin.
The Victorians thought that it was an aphrodisiac! It is probably an essential element for us, though only in trace amounts. We need 5-50 μg a day, and seafood such as plaice, prawns, oysters and shellfish in general are good sources. Roxarsone (below), an arsenic compound, is used in small amounts to stimulate growth of some farm animals. Rats and chickens on arsenic-free diets exhibit stunted growth. It seems that some people have built up immunity by eating small amounts on a regular basis. Sadly, however, arsenic poisoning plagues people living in areas like West Bengal and Bangladesh, where groundwater supplies are contaminated by arsenic.
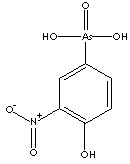
Roxarsone
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245828]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245828]