

One of the best known aromatic acetates is acetylsalicylic acid, or aspirin, which is prepared by the esterification of the phenolic hydroxyl group of salicylic acid.
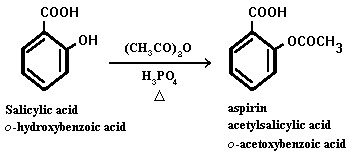
Aspirin possesses a number of properties that make it the most often recommended drug. It is an analgesic, effective in pain relief. It is also an anti-inflammatory agent, providing some relief from the swelling associated with arthritis and minor injur ies. Aspirin is also an antipyretic compound, which means it reduces fever. Each year, more than 40 million lb of aspirin is produced in the US alone, a rate that translates to about 300 tablets per year for every man, woman and child. However, it is n ot so innocuous a drug as one might imagine from its widespread use and ready availability. Repeated use may cause gastrointestinal bleeding, and large doses can provoke a host of reactions including vomiting, diarrhea, vertigo and hallucinations. The a verage dose is 0.3-1 g; single doses of 10-30 g can be fatal.
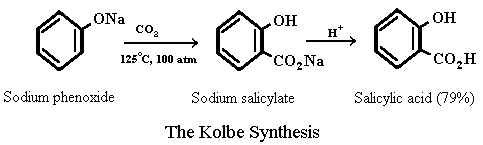
The key compound in the synthesis of aspirin, salicylic acid, is prepared from phenol by a process discovered over 100 years ago by the German chemist Hermann Kolbe. In the Kolbe synthesis (also known as the Kolbe-Schmitt reaction) sodium p henoxide is heated with CO2 under pressure and the reaction mixture is subsequently acidified to yield salicylic acid.
The following rotatable image of the aspirin molecule is presented in protein database (.pdb) format (see instructions on the Molecule of the Month Home Page for how to deal with these types of files).
References:
