|

Aspirin
The first modern pharmaceutical

Paul May
Bristol University, UK

Molecule of the Month - February 1996.
(Updated July 2016)
Also available: JSMol version.
 |
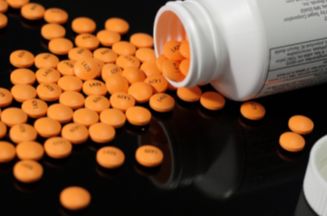
Generic aspirin tablets.
These are coated with an orange enteric coating
which prevents the tablets dissolving in the acidic
stomach causing irritation, but breaks down rapidly
in the alkaline (pH 7-9) environment present in the
small intestine. |
Is it true that aspirin is considered to be the first modern drug?
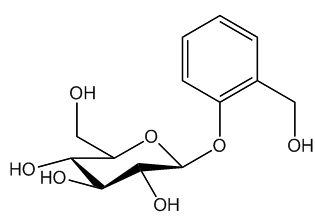
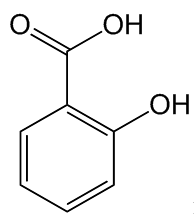
Yes, but it goes back a long way. The association of willow bark with fever-reducing properties was mentioned by the ancient Greek writer Hippocrates (440-377 BC). In the 1750s, an Oxfordshire clergyman named Edward Stone tested powdered willow bark and found it that it relieved feverish symptoms associated with malaria, publishing his discovery in 1763. Other scientists built on his observations. The molecule present in the willow, named salicin from the Latin salix, meaning willow tree, was isolated in 1828. Ten years later, it was found that salicin could be converted to salicylic acid, which had even more potent pain-relieving properties.
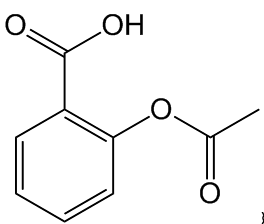
I've read the name ‘acetylsalicylic acid’ on aspirin packets. What is it and how does it differ from salicylic acid?
The common chemical name of aspirin is acetylsalicylic acid, which is the acetyl ester of salicylic acid.
 |
 |
 |
| Salicin |
Salicylic acid |
Acetyl-salicylic acid
(Aspirin) |
 So who made aspirin first?
So who made aspirin first?
What we now call aspirin, acetylsalicylic acid, was first synthesised in 1853 by Charles Frederic Gerhardt, but it was not until 1897 that chemists at the German Bayer AG company found a reliable way of making it from salicin, derived this time from the meadowsweet plant. A side-effect of salicylic acid as a medication to treat fevers and pain was that it caused gastro-intestinal complications. One version of the story says that the father of the chemist Felix Hoffmann (1868-1946, photo right) suffered from rheumatism, and that the sodium salicylate he took irritated his stomach, so the son had the bright idea of making an acetylated version of salicylic acid to try on his father. But after Hoffmann’s death, another chemist named Arthur Eichengrün claimed that he was the man responsible, and more recent analysis has supported this story. At any rate, Bayer AG named their new drug ‘aspirin’ (after the old botanical name for meadowsweet, Spiraea ulmaria), and put it on the market in 1899 as a painkiller that reduced both temperature and inflammation. Nowadays, each year around a billion aspirin tablets are taken worldwide.
Is that all that aspirin does?
It has several roles. Apart from being an analgesic (pain killer), an antipyretic (fever reducer), and an anti-inflammatory agent, there is evidence that long-term use of aspirin contributes to less risk of certain cancers, including lung, colon, prostate, bowel and breast cancers. It has also been shown that middle-aged men taking a tablet of aspirin a day can reduce the risk of heart attack by maybe 50%, as aspirin can reduce the likelihood of blood clotting.
Is aspirin safe?
Relatively safe. The toxic dose for adults is about 10-30 g, which is many times greater than the dosage in a standard tablet of ~300 mg. Both salicylic acid and aspirin can breach the stomach's protective lining, causing bleeding, but aspirin causes much less stomach irritation. Back at the time of the 1918-9 influenza (‘Spanish flu’) pandemic, aspirin was widely used as a medication, and it has been suggested that high doses of aspirin may have contributed to the fatalities. Its use with children has since been discouraged, since aspirin taken during viral illness can produce fatalities in children suffering from Reye’s syndrome.
How does aspirin work?
In 1971, the British pharmacologist Sir John Vane led the research team who showed that aspirin inhibits the production of prostaglandins (see Jan 2007 MOTM page on Prostanoic acid). These are hormones produced locally in the body when tissue is damaged or infected, affecting blood flow as they cause the disease-fighting white blood cells to race to the damage site (which is what causes the swelling and inflammation). One of these hormones, called prostaglandin E2 (PGE2), affects temperature regulation, making the body’s temperature rise and causing fever. Aspirin works by blocking prostaglandin synthesis, thereby preventing swelling, inflammation and also reducing fever.
How exactly does aspirin do this?
Prostagladins are made by an enzyme called cyclooxygenase, or COX for short. The active site on COX contains a serine group, which binds a molecule called arachadonic acid and converts it via some clever biochemistry into the required prostaglandin. However, if aspirin is present in the bloodstream it can rapidly transfer its acetyl (CH3CO) group to the serine residue in the enzyme, thus blocking the active site, and rendering the enzyme inactive.
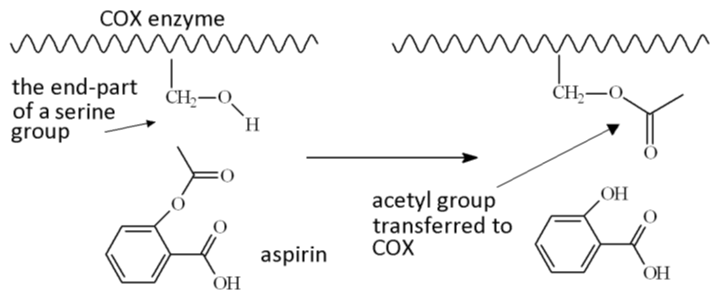
Aspirin blocks the active site on the COX enzyme by transferring its acetyl group.
In fact, there are three cyclooxygenase enzymes, COX-1, COX-2 and COX-3 (a variant of COX-1). Aspirin inhibits both COX-1 and COX-2. COX-1 occurs widely in mammalian cells; COX-2 is generated at the sites of inflammation and cell damage. It is the blocking of COX-1 that is responsible for the increased risk of stomach irritation when aspirin is taken, as it stops the formation of a protective prostaglandin (prostacyclin), in cells in the stomach lining, which reduces gastric acid production.
Does blocking COX-1 do any good at all?
Yes, certainly. Another effect of blocking COX-1 is to cut down the formation of thromboxane A2, which induces platelet aggregation and blood clotting, and this is where the cardioprotective effect of aspirin comes from.
What does aspirin do to COX-2?
COX-2 has a role in making the prostaglandin PGH2, a precursor to other prostglandins, which mediate pain, fever and inflammation. Therefore, inhibiting COX-2 reduces pain, fever and inflammation.
So can you make aspirin block COX-2 but not COX-1, so you don't get side-effects?
This was actually tried with a class of "super-aspirin" drugs. The best known of these was Vioxx (Rofecoxib), which had great promise in treating long-term arthritic complaints. It rapidly became a best-selling drug, but in 2004 it became apparent that people taking Vioxx for long periods were more likely to suffer from heart attacks and strokes, so the manufacturers withdrew it from the market.
So taking aspirin is all good?
It sometimes seems like it, but there are risks. Recent research from the University of Wisconsin has indicated that long-term use of aspirin may carry a small but significantly increased risk of age-related macular degeneration, causing loss of eyesight. It is a question of what meets the needs of a particular patient.
|
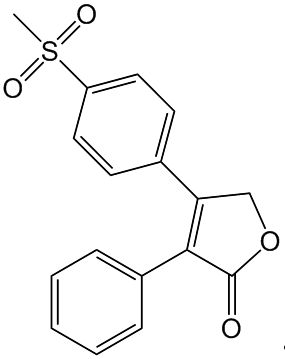
Vioxx (Rofecoxib)
|

References
- Bayer’s website about aspirin
- E. Stone, Phil. Trans. Roy. Soc., 1763, 53, 195.
- C. C. Mann and M. L. Plummer, The Aspirin Wars, A.A. Knopf, New York, 1991.
- D. Picot, P. J. Loll and R. M. Garavito, Nature, 1994, 367, 243−249
- P. J. Loll, D. Picot, and R. M. Garavito, Nat. Struct. Biol., 1995, 2, 637−643 (how aspirin deactivates prostaglandin H2 synthase).
- R. G. Kurumbail, A. M. Stevens, J. K.Gierse, J. J. McDonald, R. A. Stegeman, J. Y. Pak, D. Gildehaus, J. M. Iyashiro, T. D. Penning, K. Seibert, P. C. Isakson and W. S. Stallings, Nature, 1996, 384, 644-648 (structural basis for COX-2 inhibitors)
- C. Osborn (ed), Aspirin, Royal Society of Chemistry, London, 1998.
- J. R. Vane and R. M. Botting (eds), Therapeutic Roles of Selective COX-2 Inhibitors, William Harvey Press, London, 2001.
- W. Sneader, Brit. Med. J., 2000, 321, 1591-1594. (Discovery of aspirin: a reappraisal)
- J.R. Vane and R.M. Botting, Thrombosis Res., 2003, 110, 255–258. (how aspirin acts).
- K.D.Rainsford (ed), Aspirin and Related Drugs, Taylor & Francis, London, 2004.
- D.Jeffreys, Aspirin, The Story of a Wonder Drug, Bloomsbury, London, 2004.
- P. Sheldon, The Fall and Rise of Aspirin - the Wonder Drug, Brewin Books, Studley, 2007.
- S. Houlton, Chemistry World, December 2007, 56-59 (Vioxx withdrawal)
- T. Nesi, Poison Pills: The Untold Story of the Vioxx Drug Scandal, Thomas Dunne Books, New York, 2008.
- K. M. Starko, Clin. Infectious Dis., 2009, 49, 1405–1410 (salicylates and 1918-9 flu pandemic)
- K. M. Starko, C. G. Ray, L. B. Dominguez, W. L. Stromberg and D. F. Woodall, Pediatrics, 1980, 66, 859-864. (Reye's Syndrome and salicylate use)
- B. E. K. Klein, K. P. Howard, R. E. Gangnon, J. O. Dreyer, K. E. Lee and R. Klein, J. Am. Med. Assoc., 2012, 308, 2469-2478 (Aspirin and macular degeneration)


 Back to Molecule of the Month page [DOI:10.6084/m9.figshare.5245354]
Back to Molecule of the Month page [DOI:10.6084/m9.figshare.5245354]
![]()
![]()
![]()
![]()




 So who made aspirin first?
So who made aspirin first?
