![]()
Astaxanthin
The reason flamingoes are pink!
![]()
Catherine Cook
Sussex University, UK
![]()
Molecule of the Month June 2021
Also available: HTML version.
![]()

[Photo: Mario Grievik, used with permission.]
AstaxanthinThe reason flamingoes are pink!
Catherine Cook
Molecule of the Month June 2021
|
 [Photo: Mario Grievik, used with permission.] |
Well, shellfish, small brine shrimps to be precise. Astaxanthin is the molecule that gives fish such as salmon, trout, crustaceans and shellfish their pink/red colouration which is passed on to the flamingos when they eat them.
From eating algae.
 Ok, and the algae?
Ok, and the algae?They biosynthesise their own astaxanthin and other pigments. As the molecule travels up the food chain from algae to (shell)fish to flamingoes, their concentration in the organism increases and the colour becomes more apparent. Flamingos reared in zoos often don’t have access to the right type of shrimp eating the rght type of algae, and so turn white unless they are give food supplements containing astaxanthin to maintain their familiar pink colour!
Lobsters actually come in many different colours because of the other pigments in their shells. Most living animals that contain astaxanthin do not look very pink because it is surrounded by a protein that masks its colour. In shellfish, astaxanthin is almost exclusively found in the shells, with only low amounts in the flesh itself. But when they are cooked, most of these less-robust pigments are destroyed, the protein that masks astaxanthin denatures revealing its colour, and the lobster turns a familiar orangey-red. This is also the reason raw prawns look greyish, but turn pink when they are cooked.
Yes, astaxanthin is actually part of a family of pigment molecules called xanthophylls (meaning ‘yellow leaf’), which are chemically quite similar to each other. These are not only made by algae but by many plants as well. Xanthophylls are similar in structure to carotenes (the orange pigment found in carrots which is a precursor to Vitamin A (see MOTM for Jan 2017)), except that xanthophylls contain oxygen atoms while carotenes are purely hydrocarbons. Both groups of molecules contain extended chains of conjugated (alternating single and double) bonds, with differing chain lengths and side-groups giving rise to slightly different molecules and colours.
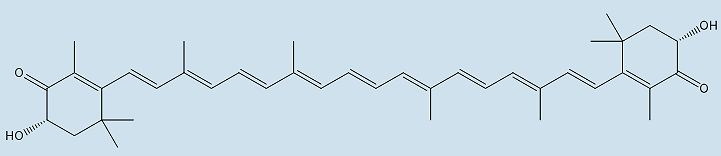 |
|
| Structure of astaxanthin | |
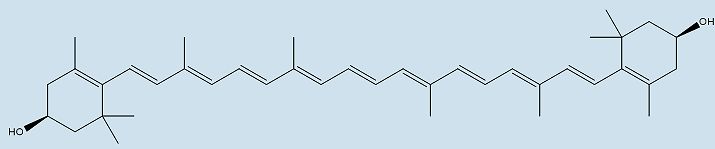 |
|
| Structure of zeaxanthin | |
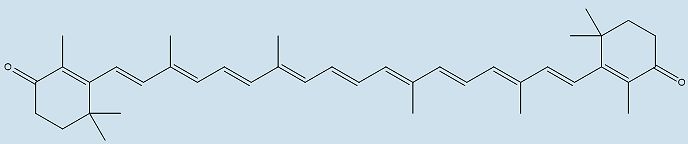 |
|
| Structure of canthaxanthin | |
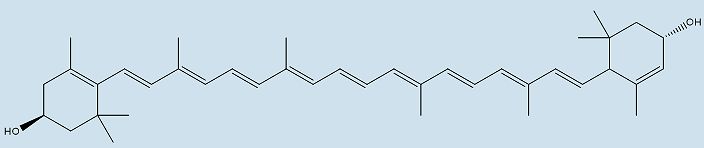 |
|
| Structure of lutein |
For example, the distinct colour of the American flamingo is made when the molecule loses the two -OH groups on either end of the astaxanthin molecule, creating canthaxanthin. Canthaxanthin is also found in some fish (e.g. The Atlantic salmon). Zeaxanthin is formed when the two carbonyl groups on either end of astaxanthin are removed, and this molecule is the yellow/red pigment found in paprika, saffron and corn maize. Another xanthophyll, lutein, is responsible for the colour of papaya, peaches, prunes, and squash. Many songbirds, such as the golden oriole, yellow warbler, and common yellowthroat, colour their feathers with lutein obtained from their diet.
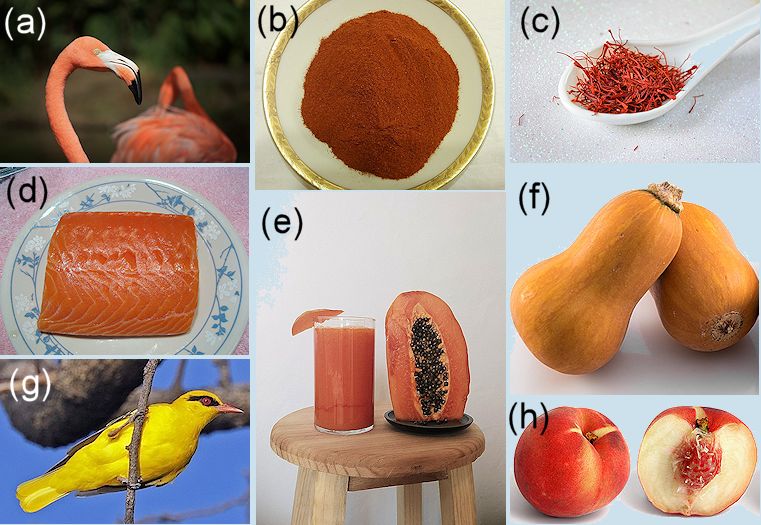
Colours in nature caused by xanthophylls.
Photos, all via Wikimedia Commons: (a) American flamingo [Hugoesteban14, CC BY-SA 4.0], (b) paprika powder [Ljiljana Sundać, CC BY-SA 4.0], (c) saffron [Salonik Saffron, CC BY-SA 4.0], (d) flesh of an Atlantic salmon [Robpedia at English Wikipedia, Public domain], (e) papaya [El Mono Español, CC BY-SA 4.0], (f) honeydew squash [Ɱ, CC BY-SA 4.0], (g) golden oriole [Charles J. Sharp, CC BY-SA 4.0].
Yes it can. The carbon atoms joined to each of the -OH groups are chiral as they have 4 different groups attached to them. This give 3 optical isomers, one where both -OH groups go into the page (R,R), one where they both point out of the page (S,S), and a final one where one -OH group points into the page and the other points out (R,S). Synthetic versions tends to contain a mixture of all 3 isomers, while the natural one has the -OH groups leaning in the same direction (3S,3’S-astaxanthin).
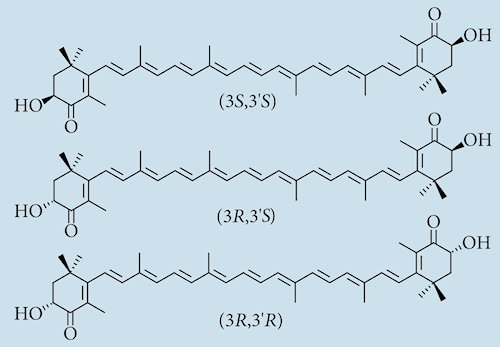
The three optical isomers of astaxanthin
As well as animals, is it used to colour other things?Yes, the xanthophylls are often used as pigments in foodstuffs and medicines, and the EU has given them each an ‘E-number’ code. They are considered to be generally safe molecules to ingest because humans and animals already consume them through the food we eat. Indeed, for chickens, the yellow colour of egg yolks, fat, and skin comes from ingested xanthophylls (mostly lutein (E161b)) added to their diet specifically to make them more yellow, and hence more appealing to the public. Canthaxanthin (E161g) has been used as an ingredient in a ‘tanning pill’ for humans, where it simulates a suntan by depositing in the fatty layer beneath the skin and imparts a range of colours, from orange to brown. However, despite many companies selling such pills, the US FDA consider them to be ‘adulterated cosmetics’ and they are illegal in many countries. Zeaxanthin (E161h) is also commonly used in small amounts as a colourant in medicines, foods, and cosmetics, but is often marketed (together with lutein) in much larger doses as a treatment for eye disorders, especially age-related macular disorder. This is because both zeaxanthin and lutein are naturally found in the macular (the yellow part of the retina), but their effectiveness in treating such eye disorders is unproven – although that hasn’t stopped the supplement manufacturers selling it anyway! |
 A fried dinner with salmon (which is often colourised using astaxanthin), fried eggs (the yolks of which are made yellower by adding lutein), and bell peppers (which naturally contain carotinoid and xanthophyll pigments). [Photo: Silar, CC BY-SA 4.0 via Wikimedia Commons] |
As a drug, astaxanthin has numerous benefits to human health such as treating Alzheimer’s and Parkinson’s diseases as well as preventing sunburn and cancer, and improving sleep, to list a few.
Simples, it is an amazing antioxidant.
An antioxidant is something that protects a organism against free radicals - uncharged molecules that contain unpaired valence electrons. For example, when an oxygen molecule, O2, splits in the body to become two O atoms, each atom will have a lone electron. Since electrons do not like to be alone, these free radicals (O atoms in this case) roam the body looking for another electron they can steal from an unsuspecting nearby molecule. But ripping an electron from an important stable biomolecule, for example DNA, can cause damage to that molecule, or to parts of the body such as proteins and cells. It is this damage that can lead to diseases such as atherosclerosis and cancer, which is why antioxidants such as astaxanthin can help prevent them. Antioxidants can act as electron donors, happy to give away electrons to any radical that wants one. This means that the free radicals can safely take their required electrons from the antioxidant molecules instead of from important biomolecules, which are now left alone and unharmed.
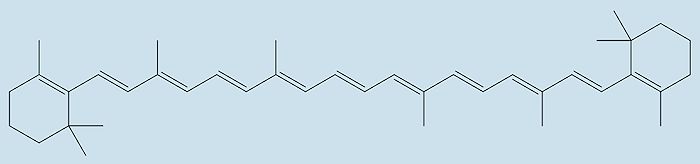
Although vitamins such as A, C & E are also powerful antioxidants, astaxanthin is better. The antioxidant activity of a molecule is based upon its conjugated chain length. The more double-bonds the better, as these contain electrons that are easily donated. Astaxanthin has 13 conjugated double-bonds whereas β-carotene (vitamin A) only has 11, making astaxanthin much more efficient (maybe as much a five times) as an antioxidant.
 |
|
| Structure of β-carotene |
Can you have too many antioxidants?Despite Sheldon from The Big Bang Theory’s worries when faced with a blueberry-pie eating contest, running out of oxidants is not what he should be concerned about. Like most things, too many antioxidants can also be bad for you, just like how too much β-carotene (from eating carrots) can turn your skin orange (see MOTM for April 2002). Like the radicals that antioxidants try to protect you from, an excessive amount of antioxidants has also been linked to an increased risk of cancer. In very high concentrations antioxidants can even reverse their purpose and become pro-oxidant. |
 [Still photo taken from a YouTube video of The Big Bang Theory: SE6E04] |
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.14531754]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.14531754]