Structure
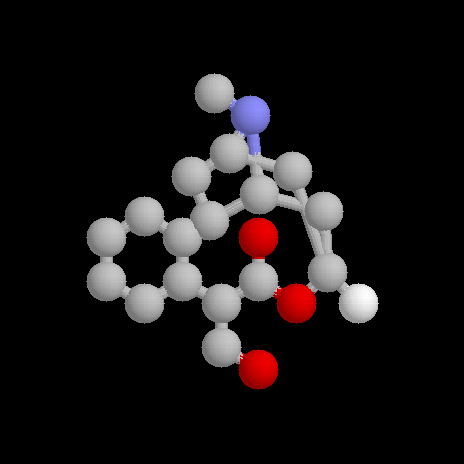
Atropine
The structure of atropine was investigated by R Willstätter in the late 1890's. It was found that on hydrolysis, atropine gave (±)-tropic acid and tropine, which was shown to be an alcohol.
Tropic acid was shown to have a molecular formula of C9H10O3 and lost a molecule of water to yield atropic acid on strong heating. Atropic acid, C9H8O2, had the structure (1) implying that tropic acid was either: compound (2) or (3):

Mackenzie and Wood showed that tropic acid was compound (2), 3-hydroxy-2-phenylpropanoic acid, by unambiguous synthesis in 1919 while Fodor, et al, demonstrated the absolute configuration by its correlation with (-)-alanine in 1961:
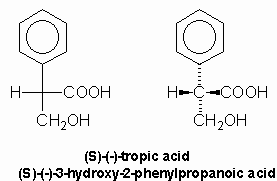
Tropine, C8H15ON contains a hydroxyl group and behaves as a saturated compound. Ladenburg (1883, 1887) demonstrated that the molecule contained a reduced pyridine nucleus. His work lead him to propose two possible structures for tropine, (4) and (5):
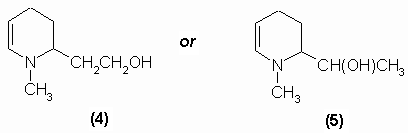
Merling (1891) obtained tropinic acid by oxidation of tropine using chromium trioxide. Tropinic acid was shown to be a dicarboxylic acid which contained the same number of carbon atoms as tropine:
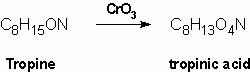
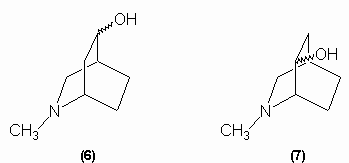
Since tropinic acid is a dicarboxylic acid and no carbon is lost in the oxidation, the alcohol group oxidation must have involved ring cleavage and so the hydroxyl group had to be within a ring. This made the Ladenburg structures untenable and so Merlin proposed either structure (6) or (7) for tropine:
Willstätter (1895 - 1891) notes that tropinone was formed during oxidation prior to ring cleavage. This substance was a ketone which was demonstrated to contain the CH2COCH2 moiety by the formation of dibenzylidene and di-oximino derivatives. This, in turn, made Merling's structures untenable and so Willstätter proposed structure (8) for tropine:
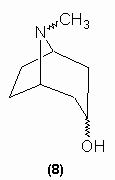
The structure of tropine has been confirmed to be (8) by synthesis by Willstätter (1900 - 1903) and by Robinson (1917) - see section on synthesis.