
![]()
Benzoyl Peroxide
(and other chemicals used
for the treatment of acne)
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month February 2017
Also available: HTML version.
![]()
  |
Benzoyl Peroxide(and other chemicals used
|
Yes, it is one of the most popular medications for treating acne, though it does not work for everyone, and the US Food and Drug Administration (FDA) provide advice.
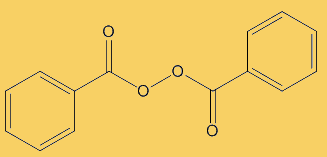
Because benzoyl peroxide is very reactive, it is effective at killing bacteria. Thus means that infected areas are cleaned up and it can nip problems in the bud before acne and inflammation develop. In contact with the skin, it decomposes to oxygen and benzoic acid, a possible equation being:
C6H5C(O)O-OC(O)C6H5 + H2O 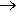 2 C6H5COOH + ½ O2
2 C6H5COOH + ½ O2
Neither of the products is toxic (benzoic acid is widely used as a food preservative).

A range of commercially available acne medications that use benzoyl peroxide.
The O-O bond in peroxides is very weak, with a bond energy ~ 90-120 kJ mol-1.
It’s been put down to inter-nuclear repulsion, coupled with repulsions between non-bonding (lone) electron pairs, similar to the reasons for the low F-F bond energy in F2 molecules. Because it is so reactive, in concentrated form benzoyl peroxide represents a safety hazard – it is said to set paper and other combustibles (e.g. wood, oil) on fire. The gels and creams used to treat acne, with concentrations in the range 2.5% to 10%, are perfectly safe from that point of view.
Photos of a teenage girl with acne before and after using a benzoyl peroxide based cream.
 What else does it do?
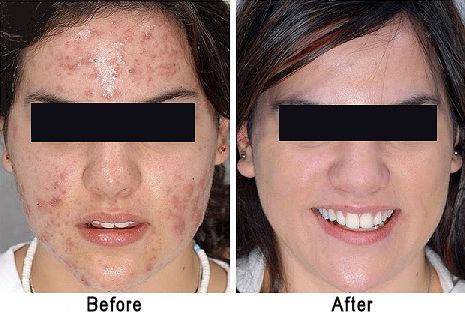
What else does it do?Benzoyl peroxide is widely used as a catalyst in the polymerisation of molecules like styrene (phenylethene) to form polystyrene, which used to make many things from drinking cups to packaging material. The polymerisation process is a free-radical chain reaction, but forming the initial free radical from stable styrene molecules is quite difficult unless extreme conditions are used. This is where adding an initiator like benzoyl peroxide comes in (see a video of this on Youtube). Breaking the weak O-O bond (homolytic fission, as the two oxygen atoms have equal electronegativities) creates two benzoyl free-radicals.
A “dot” beside an atom shows the presence of an unpaired electron there, which is what radicals are so reactive, they try to find another radical to pair up with, generating a covalent bond. These benzoyl radicals then eliminate CO2 forming phenyl radicals. Because stable molecules turn into reactive radicals, this is referred to as an initiation step, it initiates the formation of radicals.

Although only a small number of phenyl radicals are generated, once formed they can interact with a phenylethene monomer in a propagation step; one radical disappears but is replaced by another one, so radicals are “propagated” in a chain reaction.
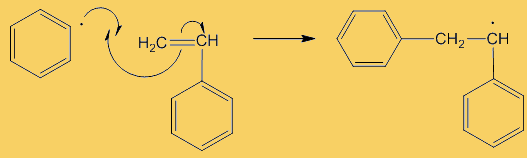
They are used to show the movement of electrons in a reaction mechanism when bonds are broken and formed. The arrows come from a source of electrons and end up where the electrons are going – usually forming a new bond.
No, it is because only one electron is involved, and is known as a “fishhook”. If two electrons were moving, it would have a full head.
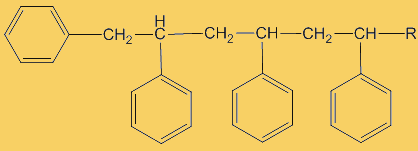
The process repeats many times. Each step uses a radical and generates one.
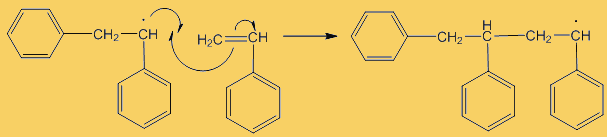
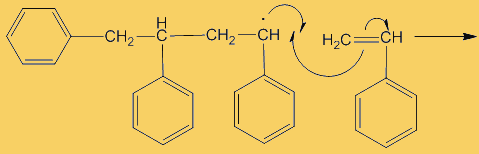
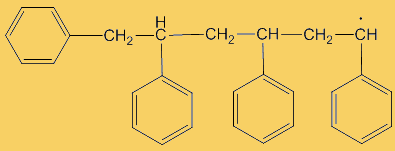
This process continues until either the monomer is all consumed, or else a termination step occurs – in practice, thousands of monomers are joined before this happens. The concentration of radicals is so low that collisions between a radical and a monomer molecule are much more frequent than collisions between two radicals. Termination steps use up all the radicals, so the reaction stops (hence the name termination); in the next equation R· refers to a non-specific radical.
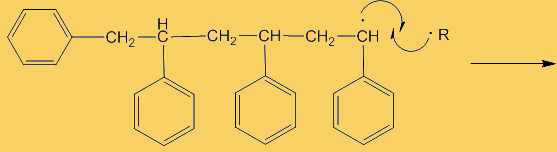
 And this is useful?
And this is useful?One familiar application for this is as the hardener in two-part wood fillers, like the one shown on the right. A styrene-based filler is added to a smaller amount of the peroxide-based hardener. They are mixed thoroughly then applied to the hole to be filled. It rapidly hardens and can then be sanded smooth. It's worth wearing gloves and eye protection while handling it though!
Salicylic acid (2-hydroxybenzoic acid) is also used.
|
It is apparently very good at cleaning out the pores, and it also has anti-inflammatory properties (it is the active ingredient of aspirin).
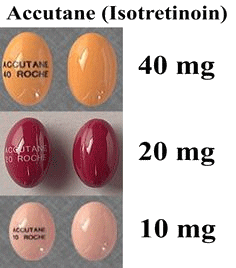
Well, there’s 13-cis-retinoic acid, marketed under names like Isotretinoin, Accutane or Roaccutane, usually in the form of pills (photo below, right) or creams.
|
 Although this substance has been widely used for many years, it has been claimed that one side-effect can be inflammatory bowel disease, but a survey has found no basis for this. More worrying is the possibility that for some people taking this medication can result in them having mood changes – or worse. Current advice from the (UK) National Health Service is: “There have been reports of people experiencing mood changes while taking isotretinoin. There is no evidence that these mood changes were the result of the medication. However, as a precaution, contact your doctor immediately if you feel depressed or anxious, have feelings of aggression or suicidal thoughts.”
One case that gives rise to concern is a 2002 plane crash in Florida. A 15-year old youth named Charles J. Bishop stole a Cessna 172 aircraft and crashed it into the Bank of America tower in Tampa, Florida, killing himself. The pilot’s mother took out a lawsuit against the manufacturers of Accutane, which she blamed for her son’s actions, though she dropped the suit 5 years later. Nevertheless, in the US there is now a 'Roaccutane suicide help-line', and a rash (excuse the pun) of lawyers who are willing to make a claim against the manufacturers of these medications for alleged side-effects.
Although this substance has been widely used for many years, it has been claimed that one side-effect can be inflammatory bowel disease, but a survey has found no basis for this. More worrying is the possibility that for some people taking this medication can result in them having mood changes – or worse. Current advice from the (UK) National Health Service is: “There have been reports of people experiencing mood changes while taking isotretinoin. There is no evidence that these mood changes were the result of the medication. However, as a precaution, contact your doctor immediately if you feel depressed or anxious, have feelings of aggression or suicidal thoughts.”
One case that gives rise to concern is a 2002 plane crash in Florida. A 15-year old youth named Charles J. Bishop stole a Cessna 172 aircraft and crashed it into the Bank of America tower in Tampa, Florida, killing himself. The pilot’s mother took out a lawsuit against the manufacturers of Accutane, which she blamed for her son’s actions, though she dropped the suit 5 years later. Nevertheless, in the US there is now a 'Roaccutane suicide help-line', and a rash (excuse the pun) of lawyers who are willing to make a claim against the manufacturers of these medications for alleged side-effects.
More serious is the risk to women taking the drug. Like thalidomide (MOTM July 2000) it is a teratogen, producing birth defects in babies born to pregnant women who took it. These effects were first noticed in 1983, a year after the introduction of the drug. The body mistakes it for retinoic acid, used in embryo development. In 1988, the FDA estimated that between 1982 and 1986 it caused the birth of 900 to 1,300 babies with "severe birth defects". It was also linked with up to 1,000 spontaneous abortions and estimated that some 5,000 to 7,000 women had induced abortions "solely because of Accutane exposure and fear of birth defects".
A pregnancy prevention programme, iPLEDGE, now operates in the USA, with the aim of stopping pregnancies during isotretinoin treatment. Before starting treatment in most countries special consent is needed before the drug can be used, and it cannot be taken during pregnancy or in the following month, nor are isotretinoin users allowed to be blood donors because of the risk to the foetus in any pregnant woman receiving the transfusion.
![]()
![]()