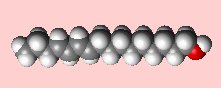
(10E,12Z)-hexadeca-10,12-dien-1-ol
![]()
Bombykol
The sex pheromone
of the silk moth
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Molecule of the Month May 2009
Also available: JSMol version.
![]()
 (10E,12Z)-hexadeca-10,12-dien-1-ol |
BombykolThe sex pheromone
|
Bombykol... sounds explosive?Not at all. In fact, it is the sex pheromone of the silk moth. Sort of make love, not war?Yes, you got it. The "bomb" bit comes from the name of the silk moth, Bombyx mori.
|
|
 So he discovered pheromones?
So he discovered pheromones? Yes and no. He was the first person to determine the structure of a pheromone, but the first real indications of the existence of insect pheromones were obtained by Jean-Henri Casimir Fabre (1823-1915), doyen of French entomologists. A photo of him is shown on the right, and he also appeared on a French stamp, left.
Yes and no. He was the first person to determine the structure of a pheromone, but the first real indications of the existence of insect pheromones were obtained by Jean-Henri Casimir Fabre (1823-1915), doyen of French entomologists. A photo of him is shown on the right, and he also appeared on a French stamp, left.
Fabre's birthplace at Saint-Léons in the Aveyron département is now the site of Micropolis, la cité des insectes, a tourist attraction all about entomology; there is also a museum on Fabre's life at Saint-Léons.
 "L'Harmas", his house at Sérignan (photo, right), in the Vaucluse northeast of Orange, was well screened by trees. In a series of key experiments, initially studying the Great Peacock Moth, Fabre found that a female moth could attract males over large distances, even on stormy nights ("under conditions in which not even an owl would venture out").
"L'Harmas", his house at Sérignan (photo, right), in the Vaucluse northeast of Orange, was well screened by trees. In a series of key experiments, initially studying the Great Peacock Moth, Fabre found that a female moth could attract males over large distances, even on stormy nights ("under conditions in which not even an owl would venture out").
He deduced that the male antennae had something to so with it, noted that surrounding the female with trays of molecules like naphthalene or lavender oil did not deflect the males from their aim, and observed that males were attracted to an empty cage where the female had spent the previous evening. "Whatever she touched, above all with her fat belly apparently, has become impregnated, as a result of long contact, with certain emanations. There you have her bait, her love-philtre."
"It is smell, therefore, that guides the Moths, that gives them information at a distance".
By Butenandt's time, science had come a long way since Fabre, but he did not have sophisticated techniques for identifying very small quantities of molecules, or for their separation, that scientists now take for granted. He also had the problem of obtaining the quantities of the insects needed, of handling tiny quantities of chemical extracted, and how to test whether he had separated the right chemical. Not to mention the intervention of World War II.
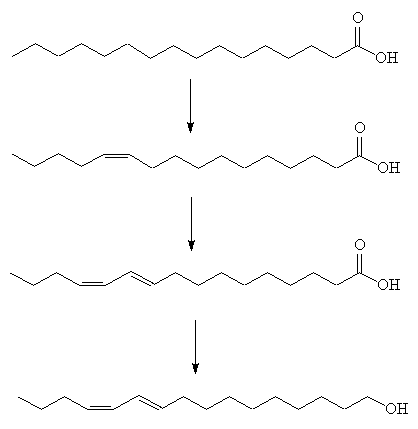
The female moth makes it from hexadecanoic acid. This is made by the usual method of carboxylic acid synthesis, using acetyl coenzyme A.
 |
 |
| Hexadecanoic acid | Spacefill representation |
The saturated acid first undergoes oxidation catalysed by a Z11 desaturase enzyme, to generate the first double bond. The second step is more unusual, involving 1,4-elimination of two hydrogens to afford a conjugated diene, catalysed by a 10,12-desaturase enzyme. Finally a reductase enzyme converts the carboxylic acid group into a primary alcohol (fatty acyl reduction).
At the time, there was a flourishing silk industry in Europe, and the moths were easy to obtain in Germany. After World War II, the German silk industry collapsed, and Butenandt had to get them from further afield, even Japan.
He had to extract the pheromone. This is found in glands at the end of the (female) abdomen. In his final experiments in 1956 he obtained the pheromone from 500,000 Japanese silk moths, and this gave him 6.4 milligrams of pure bombykol.
He did this by bioassays. Unlike the wild silk moth (Bombyx mandarina), the domesticated silk moth cannot fly, but responds to the female's pheromone by getting excited and flapping his wings in what is known as a "flutter dance". The scientists tested each fraction by diluting it and finding out the minimum concentration needed to make 50% of the males in a sample respond. The purer the material, the less needed to produce the flutter dance. Eventually material was obtained that contained just one component, and was pure bombykol. The team were lucky in that the pheromone only comprised one substance; multi-component pheromones are common (although the female Bombyx mori emits a mixture comprising bombykol with a small amount of the corresponding aldehyde, bombykal, the male receptor only bonds bombykol).

Male silk moth, right, gets excited by the female. The female is bigger, because of the eggs she is carrying.
She is extending her pheromone gland and releasing bombykol.
Reproduced with the permission of Professor Walter S. Leal.
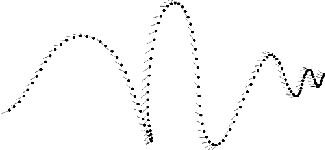 How do male moths find the female?
How do male moths find the female?In Butenandt's time, people knew that male moths would encounter a female moth's pheromone by crossing downwind of the female, then flying towards the female in a zigzag path up the plume of the female's pheromone, using its large antennae to sense it, and narrowing its zigzag as it got nearer to the female and as the concentration of pheromone increased (see image, right). In the case of the flightless Bombyx mori, she stays put and waits for a male to come to her.
Bombykol molecules diffuse through open pores in the hairs on the moth's antennae and reach a receptor protein in the membrane. This produces an electrical change leading to a nerve impulse being sent to the brain.
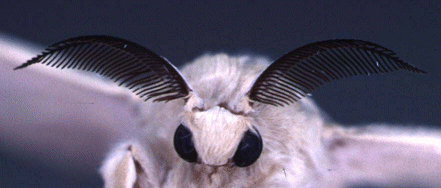
A silk moth's antennae.
Reproduced with the permission of Professor Walter S. Leal.
A German biologist named Dietrich Schneider found a way of measuring the response of the antenna to pheromones (1957). Postulating that when an antenna detected a pheromone molecule, it might produce an electrical response much as nerve cells did, he removed the antenna from a male Bombyx mori and placed it in a salt solution (to keep the cells fresh). He then connected it in a circuit between two electrodes, and blew an air current containing bombykol molecules over the electrode, which did immediately respond with a pulse of electricity.
We're only just getting started. For a start, the antennae have to detect one molecule in the presence of a lot of different ones, and also in very, very, small amounts.
It's reckoned that it can detect female moths over 10 km away, sensing bombykol at a concentration as low as 1 molecule per 1017 air molecules. It detects individual molecules but it is thought that a moth has to detect around 200-300 bombykol molecules in a second to produce a behavioural response.
Not yet. The bombykol molecule has a long carbon chain, making it pretty hydrophobic. It has to cross from the sensillar wall (where it docks in the antenna) across the aqueous solution that makes up the sensillar lymph, to reach the odorant receptor.
More chemistry. A pheromone binding protein (PBP) binds the bombykol in a pocket and transports it to the receptor.
It has to hold the bombykol fairly weakly, so that the bombykol can be released at the receptor, so it's not a question of strong covalent bonds. The protein holds the bombykol in a hydrophobic pocket; it looks as if the forces are mainly weak hydrophobic (Van der Waals') type forces, though there is one hydrogen bond involving the OH group. The pocket in the PBP that binds the bombykol is flexible. It will accommodate non-pheromone molecules, such as 1-iodohexadecane (1 molecule) or the bell pepper odorant 2-isobutyl-3-methoxypyrazine (2 molecules), but with much lower affinity. Possibly these weakly held molecules would be dropped from the PBP on its journey and "filtered out". It's also not just a matter of transporting the pheromone, protecting the pheromone comes into it too.
The lymph fluid contains enzymes that break down the pheromone.
When the bombykol molecule has passed on its message to the receptor, it has to be removed very fast and deactivated. This is in order that fresh messages can be received and passed on, so that the flying moth can change course. Otherwise the receptor would be "jammed on".
When it gets to the receptor, the protein undergoes a conformational change, due to a decrease in pH, that weakens its hold on the bombykol, causing the PBP to unfold and release the bombykol.
Once the male has found the female, they mate, possibly for up to a day; now that the eggs are fertilised, the female soon commences laying. Once she has done that, she will die, but the fertilised eggs will hatch and give rise to more silk worms (and moths). The pheromone has done its job.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255035]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255035]