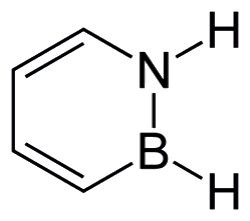
Borazine
The inorganic benzene

William Griffiths and Stephen Belding
Rugby School, UK

Molecule of the Month March 2025
Also available: JSMol version.

|
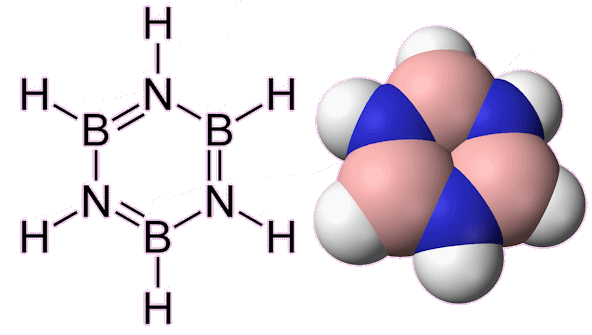
Borazine: Structure and spacefill model.
[Images: Benjah-bmm27, Public domain, via Wikimedia Commons,
Ben Mills, Public domain, via Wikimedia Commons] |
Borazine - sounds boring...
Not at all! The name comes from the fact it is made from the element boron. Borazine, also sometimes known as borazole, (B3N3H6) is actually a fascinating molecule commonly referred to as ‘inorganic benzene’. The molecule is isoelectronic and isostructural with benzene and the molecules share similar physical properties. Borazine is a stable, colourless liquid with a boiling point of 55 °C, a melting point of -58 °C, and an aromatic odour. Unlike benzene, which consists of a ring of six carbon atoms, borazine features a six-membered planar ring composed of alternating boron and nitrogen atoms. This unique structure imparts distinct properties to borazine, making it useful in various applications (Housecroft et al., 2018).
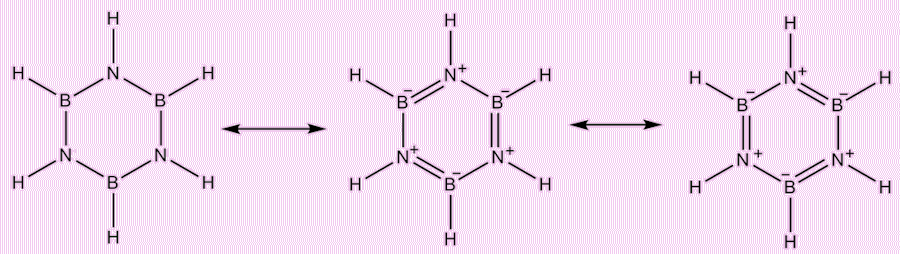
Borazine in its canonical form.
[Image: Adam Rędzikowski, Public domain, via Wikimedia Commons]
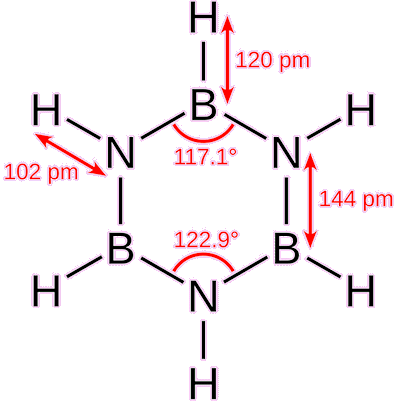 Borazine’s bond angles and lengths.
Borazine’s bond angles and lengths.
[Image: Hbf878, Public domain, via Wikimedia Commons]
|
So, it’s Like benzene… but inorganic?
As it is isostructural (having an analogous structure) and isoelectronic (having the same number of electrons) with benzene (C6H6), borazine is often called 'inorganic benzene'. Borazine also shares the property with benzene, that its bond lengths within the ring are all equal (Housecroft et al., 2018). X-ray crystallographic methods have shown this to equal 1.429 Å (Boese et al., 1994). However, the term ‘inorganic benzene’ is not entirely accurate because the electronegativities of boron and nitrogen differ significantly: 2.04 for boron and 3.04 for nitrogen on the Pauling scale. An effect of this is that borazine is not a perfect hexagon, with bond angles of 117.1° at the boron atoms and 122.9° at the nitrogen atoms (Baranac-Stojanović and Stojanović, 2013).
|
If it’s like benzene, is it also aromatic?
The aromaticity of borazine has been a subject of much debate among chemists. Borazine, like benzene, is a planar molecule, meaning it has a flat structure. It has conjugated bonds, which means that the electrons in its bonds can be shared across the entire molecule. This allows borazine to undergo electrophilic substitution reactions — a key feature of aromatic compounds (more details below).
In borazine, delocalisation of p-electrons forms a ring system. Each nitrogen atom donates two electrons to an empty p-orbital on the boron atoms, similar to how benzene’s structure works (Gregory, 2000). Although the rule was originally developed for hydrocarbons, borazine also satisfies Hückel's rule (4n + 2), which is used to predict aromatic character. This suggests that borazine could be aromatic.
However, borazine has poorer electron delocalization than benzene. This is because the difference in electronegativity between boron and nitrogen causes the B-N bonds to have some ionic character, meaning they don’t share electrons as evenly. Because of this, some studies argue that borazine is not truly aromatic.
One way to measure aromaticity is through aromatic stabilization energy (ASE). This measures the difference in energy between the actual molecule and a hypothetical molecule that doesn’t have any electron delocalization. A lower energy for the delocalized molecule indicates it is more stable compared to the non-delocalized version. |
| 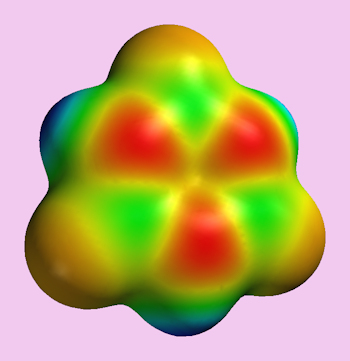
A representation of borazine’s electron density/distribution.
[Image: Ben Mills, Public domain, via Wikimedia Commons] |
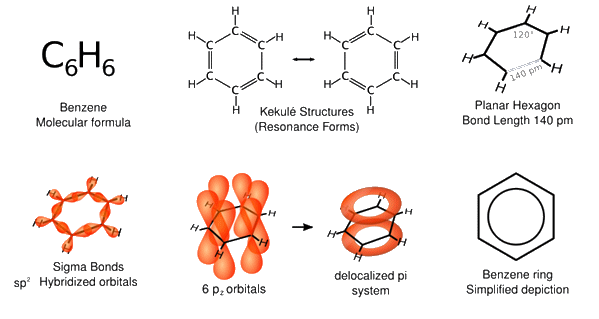
Various representations of benzene.
[Image: Vladsinger, Public domain, via Wikimedia Commons]
|
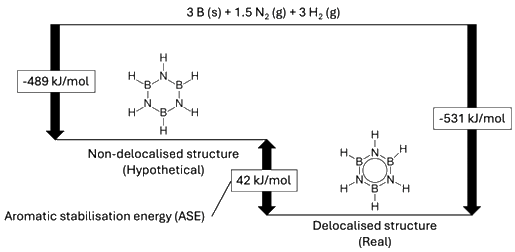
Energy-level diagram showing the standard enthalpy of formations of borazine
and the hypothetical borazine structure without any delocalized electrons.
The delocalized form is more stable by 42 kJ/mol.
[Image: Self-drawn; Non-delocalised structure: Vchorozopoulos, Public domain, via Wikimedia Commons;
Delocalised structure: Benjah-bmm27, Public domain, via Wikimedia Commons;
Data: Islas et al., 2007, Karpagam Academy of Higher Education, 2018]
|
For comparison:
- The aromatic stabilization energy of benzene is approximately 151 kJ/mol.
- The aromatic stabilization energy of borazine is only about 42 kJ/mol (Islas et al., 2007; Báez-Grez et al., 2022).
It has been reported that borazine has approximately 28% of the aromaticity of benzene, but the ring-current patterns (an effect aromatic molecules show when subjected to a magnetic field) cannot be clearly identified (Báez-Grez and Pino-Rios, 2022).
In summary, while borazine has some characteristics of aromatic compounds, its differences in electron delocalization and bond nature make its aromaticity a debated topic in chemistry.
Who discovered borazine and how did they make it?
Borazine was first synthesized in 1926 by Alfred Stock (photo, right) and Erich Pohland. They produced it by reacting diborane (B2H6) with ammonia (NH3), resulting in a cyclic compound with alternating boron and nitrogen atoms, along with the release of hydrogen gas (Stock and Pohland, 1926). The reaction occurs at temperatures between 250 and 300 °C:
3 B2H6(g) + 6 NH₃(g) 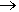 2 B3N3H6(l) + 12 H2(g) 2 B3N3H6(l) + 12 H2(g)
Under certain conditions, this reaction proceeds via ammonia borane (H3NBH3), often highlighted in A-level Chemistry as a dative-bond example, where nitrogen donates its lone pair to boron. Once primarily of academic interest, ammonia borane is now explored as a hydrogen-storage medium for fuel, given its stability at ambient conditions and high hydrogen density (greater than liquid hydrogen) (Stephens et al., 2007).
|
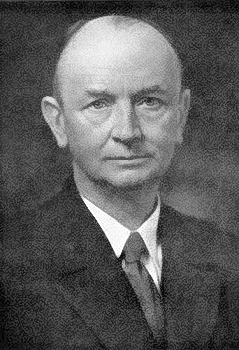
Alfred Stock (1876-1946)
[Image: Sebastian Ot., Public domain, via Wikimedia Commons]
|
 |
 |
Diborane
[Image: Ben Mills, Public domain, Wikimedia Commons] |
Ammonia-borane
[Image: Ben Mills, Public domain, Wikimedia Commons] |
In what types of reactions does borazine participate?
Electrophilic Substitution – Contrary to earlier beliefs, borazine does undergo reactions via electrophilic substitution, similar to benzene. Electrophiles such as (CH3)2F+, (CH3)2CH+, and (CH3)3C+ can react with borazine. However, other electrophilic substitution reactions, such as nitration, have not been observed to occur (Chiavarino et al., 1998).
Electrophilic Addition – Unlike benzene, borazine can also participate in electrophilic addition reactions (which are even favoured over substitution reactions!). For instance, hydrogen chloride (HCl) or hydrogen bromide (HBr) can add across the borazine ring, with the Cl- or Br- ions attaching to the electron-deficient boron atoms, rather than to the electron rich nitrogens.
(HBNH)3(l) + 3HCl(aq) 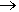 (ClHBNH2)3(s)
(ClHBNH2)3(s)
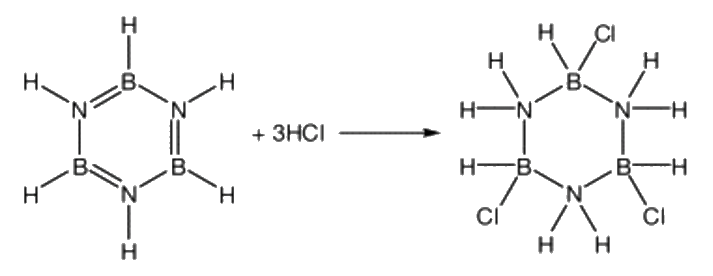
[Image: Rasmussen et al., 2001]
The product of this reaction possesses a chair conformation, similar to cyclohexane. Borazine can be reformed via treatment with sodium borohydride (NaBH4) (Housecroft et al., 2018).
Borazine can also react with halogens, such as three molecules of bromine (Br2), to form B-tribromo-N-tribromoborazine. A catalyst is not needed for this reaction.
Hydrolysis: Borazine can undergo hydrolysis under mild conditions, producing boric acid (B(OH)3), ammonia (NH3), and hydrogen gas (H2). The hydrolysis reaction can be represented as follows:
B3N3H6(l) + 9 H2O(l) 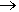 3 B(OH)3(aq) + 3 NH3(g) + 3 H2(g) 3 B(OH)3(aq) + 3 NH3(g) + 3 H2(g)
Polymerization: The polymerization of borazine into polyborazylene (structure, right) is accompanied by a dehydrogenation reaction. This reaction occurs at relatively low temperatures, typically between 70°C and 110°C (Rasmussen et al., 2001).
n B3N3H6(l) 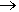 (1/n)[B3N3H4](s) (1/n)[B3N3H4](s)
|

The repeat unit for polyborazylene.
[Image: Hbf878, Public domain, via Wikimedia Commons] |
What are the derivatives of borazine?
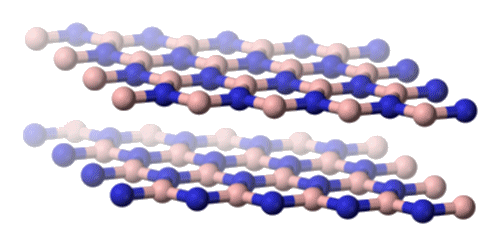
Hexagon boron nitride.
[Image: Benjah-bmm27, Public domain, via Wikimedia Commons] |
Hexagonal boron nitride (h-BN): Like graphite, h-BN consists of layers of hexagonal rings. It ranks around 2 (out of 10) on the Mohs hardness scale (Petrescu and Balint, 2007) – similar to graphite. h-BN is typically produced by the thermal decomposition of borazine at high temperatures. Compared to graphite, h-BN is a poorer electrical conductor due to its slightly ionic character, which restricts the mobility of electrons. Due to its thermal stability, h-BN was found to be an excellent lubricant in high-temperature environments, such as in jet engines and high-speed bearings. This application highlighted the practical utility of borazine derivatives in extreme conditions (Kimura et al., 1999). |
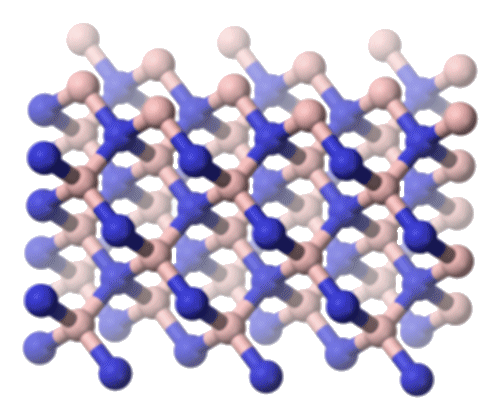
Cubic boron nitride.
[Image: Benjah-bmm27, Public domain, via Wikimedia Commons] |
Cubic boron nitride (c-BN): Analogous to diamond, c-BN is very hard, ranking around 9.5 on the Mohs hardness scale, compared to 10 for diamond. c-BN is synthesized through high-pressure, high-temperature processes using h-BN. c-BN’s hardness and ability to maintain its shape at high cutting temperatures allows it to be used as a cutting tool and abrasive, capable of cutting through hardened steels and superalloys (Poulachon et al., 2004). |
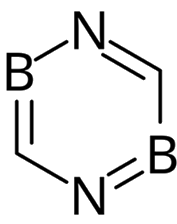
Are there any other compounds similar to borazine?
Yes! Several six-membered rings contain varying combinations of carbon, nitrogen, boron, and hydrogen atoms. Notable examples include 1,2-dihydro-1,2-azaborine (C4H6BN) and carborazine (C2H2B2N2) (Bonifazi et al., 2015). These compounds exhibit unique properties and reactivities that make them interesting for various applications.
 |
 |
1,2-Dihydro-1,2-azaborine
[Image: Ben Mills, Public domain, via Wikimedia Commons] |
Carborazine
[Image: Edgar181, Public domain, via Wikimedia Commons] |
Conclusion
Borazine, with its intriguing properties and similarities to benzene, is a fascinating molecule in the realm of inorganic chemistry. It offers valuable insights into material science and industrial applications. Its distinctive structure, reactivity, and wide range of applications make it an important subject for study and a promising candidate for future research.

Bibliography
- Báez-Grez, R. and Pino-Rios, R., 2022. The hidden aromaticity in borazine. RSC Advances, 12(8), pp.7906-7910. doi:10.1039/D1RA06457F.
- Baranac-Stojanovic, M. and Stojanovic, M., 2013. Substituent effects on cyclic electron delocalization in symmetric B- and N-trisubstituted borazine derivatives. RSC Advances, 3(46), pp.24108-24117.
- Boese, R., Maulitz, A.H. and Stellberg, P., 1994. Solid-state Borazine: Does it Deserve to be Entitled “Inorganic Benzene”? Chemische Berichte, 127(10), pp.1887-1889.
- Bonifazi, D., Fasano, F., Lorenzo-Garcia, M.M., Marinelli, D., Oubaha, H. and Tasseroul, J., 2015. Boron–nitrogen doped carbon scaffolding: organic chemistry, self-assembly and materials applications of borazine and its derivatives. Chemical Communications, 51(83), pp.15222-15236.
- Chatterjee, S., Kim, M.J., Zakharov, D.N., Kim, M.S., Stach, E.A., Maruyama, B. and Sneddon, L.G., 2012. Syntheses of boron nitride nanotubes from borazine and decaborane molecular precursors by catalytic chemical vapor deposition with a floating nickel catalyst. Chemistry of Materials, 24(15), pp.2872-2879.
- Chiavarino, B., Crestoni, M.E. and Fornarini, S., 1999. Electrophilic substitution of gaseous borazine. Journal of the American Chemical Society, 121(11), pp.2619-2620.
- Gregory, K., 2000. Organic chemistry solutions: #17.
- Grimes, R.N., 2016. Carboranes. 3rd ed. Academic Press.
- Housecroft, C.E. and Sharpe, A.G., 2018. Inorganic Chemistry. 5th ed. Harlow: Pearson.
- Islas, R., Chamorro, E., Robles, J., Heine, T., Santos, J.C. and Merino, G., 2007. Borazine: to be or not to be aromatic. Structural Chemistry, 18(6), pp.833-839.
- Karpagam Academy of Higher Education, 2018. Unit: V (p-block Elements).
- Kimura, Y., Wakabayashi, T., Okada, K., Wada, T. and Nishikawa, H., 1999. Boron nitride as a lubricant additive. Wear, 232(2), pp.199-206.
- Li, M., Wang, M., Hou, X., Zhan, Z., Wang, H., Fu, H., Lin, C., Fu, L., Jiang, N. and Yu, J., 2020. Highly thermal conductive and electrical insulating polymer composites with boron nitride. Composites Part B: Engineering, 184, p.107746.
- Petrescu, M.I. and Balint, M., 2007. Structure and properties modifications in boron nitride. Part II: Hardness modification induced by the polymorphic transformations. U.P.B. Sci. Bull., Series B, 69(1), pp.43-52.
- Poulachon, G., Bandyopadhyay, B.P., Jawahir, I.S., Pheulpin, S. and Seguin, E., 2004. Wear behavior of CBN tools while turning various hardened
- steels. Wear, 256(3-4), pp.302-310.
- Rasmussen, R.L., Morse, J.G. and Morse, K.W., 2001. Main group elements. In: Encyclopedia of Physical Science and Technology. 3rd ed. Academic Press, pp.1-30.
- Stephens, F.H., Pons, V. and Baker, R.T., 2007. Ammonia–Borane: The Hydrogen Source par excellence? Dalton Transactions, 2007(25), pp.2613-2626. doi:10.1039/b703053c.
- Stock, A. and Pohland, E., 1926. Borwasserstoffe, IX.: B3N3H6. Berichte der Deutschen Chemischen Gesellschaft, 59(9), pp.2215-2223.


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.27910497]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.27910497]
![]()
![]()
![]()
![]()


 Borazine’s bond angles and lengths.
Borazine’s bond angles and lengths.


 2 B3N3H6(l) + 12 H2(g)
2 B3N3H6(l) + 12 H2(g)