![]()
 |
BOTULINUM TOXIN(Botox)The anti-wrinkle treatment that's the most powerful neurotoxin known. |
![]()
Guillermo Godino Sedano
King´s College School, Tres Cantos, near Madrid, Spain
![]()
Molecule of the Month February 2012
Also available: JSMol version.
![]()
Botulinum toxin is the most powerful neurotoxin known to date. Only one single molecule of it is needed to stop one neuron working. This makes the lethal dose for a mouse only 1.2 ng! The table below shows the amount of various substances required to kill a normal human being. In fact, 1 gramme of botulinum toxin would be enough to kill 14,000 people (if ingested), 1.25 million people if inhaled, or a staggering 8.3 million people if injected!
|
 |
 Botulinum toxins are often found in poorly preserved food, or even in flesh wounds, and have been known since the 18th century when the German medic, Justinus Kerner (right) described botulinum toxin as a "sausage poison" and "fatty poison", because is arose from improperly handled or prepared meat products. In fact, Kerner coined the name botulism (from Latin botulus meaning "sausage"). In 1897 a Belgian scientist named Van Ermengem found that the toxin in the blood of a patient with Botulism was actually produced by a bacteria, Clostridium Botulinum. Later it was found that 4 types of bacteria can produce this toxin, Clostridium botulinum, C. butyricum, C. baratii and C. argentinense, and there are 7 distinct types of the toxin known, labelled A through G, most have several subtypes also.
Botulinum toxins are often found in poorly preserved food, or even in flesh wounds, and have been known since the 18th century when the German medic, Justinus Kerner (right) described botulinum toxin as a "sausage poison" and "fatty poison", because is arose from improperly handled or prepared meat products. In fact, Kerner coined the name botulism (from Latin botulus meaning "sausage"). In 1897 a Belgian scientist named Van Ermengem found that the toxin in the blood of a patient with Botulism was actually produced by a bacteria, Clostridium Botulinum. Later it was found that 4 types of bacteria can produce this toxin, Clostridium botulinum, C. butyricum, C. baratii and C. argentinense, and there are 7 distinct types of the toxin known, labelled A through G, most have several subtypes also.
The Clostridium botulinum bacteria responsible
for the production of Botulinum toxin.
The Botulinum toxins are proteins consisting of a sequence of ~1,300 amino acids - nearly 21,000 atoms. They can be made as a single-chain polypeptide, but in this form they are not very toxic. They are made more potent and toxic in two steps. In the first step, which usually occurs in the bacteria, the chain is cleaved into larger chain connected to a smaller chain associated with a single zinc atom. The two chains are joined by a single disulfide bond. In the second step, which occurs within the body during the neuro-toxic mechanism, the disulfide bond is reduced, which activates the toxin.
|
|
The bacteria produce Botulinum Toxin (BTX) by anaerobic respiration, so it commonly occurs in canned-food containers, where there is no oxygen. When ingested, BTX causes botulism, a serious illness which begins by causing the paralysis of the face muscles and then the paralysis of most of the body. When the muscles which control breathing become paralysed, this results in repiratory arrest and death. The bacteria can be killed and the toxin detroyed with high pressures or high temperatures (boiling for a few minutes), for example when cooking food.
Although it is very toxic, poisoning from botulism in the modern world is quite rare. Between 1990 and 2000, the Centers for Disease Control in the US reported 263 individual cases from 160 foodborne botulism 'events' in the United States, of which only 4% proved fatal. 39% occurred in Alaska as a result of traditional Alaska native foods (seal meat, etc). In the other 49 states, home-canned food was implicated in 70 (91%) events, with canned asparagus being the most numerous cause. Most US states had an incidence rate of 1 case per ten million people or less.
There are antitoxins for BTX, but it has to be used very quickly after the bacteria are ingnested, or it won´t be able to save the person.
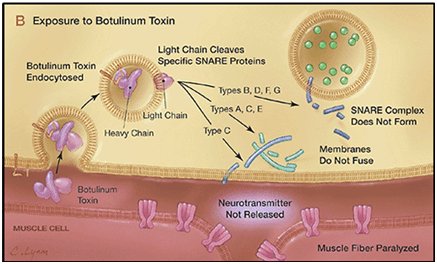
BTX works by preventing signals from the nerve cells from reaching muscles, effectively leaving the muscles without 'instructions' and so paralysed. A nerve cell contains spherical capsules called synaptic vesicles which contain a neurotransmitter molecule such as acetylcholine. When acting normally, an electrical signal passing down the nerve towards a muscle fibre causes these vescicles to move towards the interface between the nerve and muscle, and also to produce special types of protein called SNARE proteins. Three types of SNARE proteins are involved, called SNAP-25, Synaptobrevin and Syntaxin. These SNARE proteins can break through the membrane barrier at the end of the nerve cell, allowing the neurotransmitter to flood out into the receptors on the muscle, causing it to contract (see image A below).
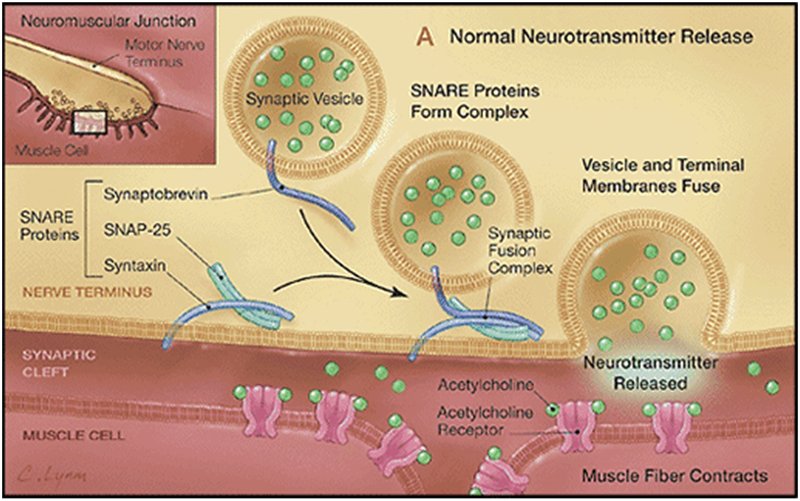
On exposure to BTX, the two chains split and the heavy chain lodges withinthe vescicles, while the lighter chain sits on the outside of the vesicle and cleaves the SNARE proteins. Different versions of BTX cleave different SNARE proteins.
But since all 3 SNARE proteins are required for a successful transmission of the signal, breaking any one of these prevents the muscle receiving the signal (as can be seen in image B below).
 Beneficial uses (Botox)
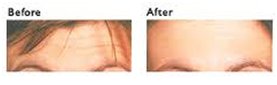
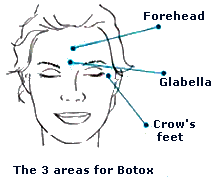
Beneficial uses (Botox)Due to its paralysing effect on muscles, BTX can be used in very small, localised doses to help certain medical ailments. In this form, the toxin is commonly known as Botox (OnabotulinumtoxinA). Botox contains some BTX, but in extremely small quantities; otherwise, most of the users of Botox would be dead! It was first used for medical purposes in 1981, to treat strabismus (Crossed eyes), by paralysing the muscle on one side of the eye that was pulling the eye into a crooked position. It is also used as a treatment for achalasia (a swallowing disorder); it works well in about 65% of the treated people. Botox also has cosmetic uses and is mainly used to relax the muscles that cause wrinkles, so the wrinkles disappear. It is also used to treat many other conditions, such as blepharospasm (excessive blinking), hyperhidrosis (excessive sweating), and migraine.
There are only 7 companies with permission to manufacture BTX for medical uses, and many more companies which can produce it for labs and for schools (for research). But there are even more which produce it illegally; they don´t have quality control, so they are able to produce the toxin with too high concentration. The products produced with this BTX are poisonous and they can kill the customers. The illegal BTX can also have concentrations which are too weak, and therefore won´t have any effect.
There is an even worse potential use for the toxin: terrorism. Botulinum Toxins are the most toxic substances known, and for this reason they were among the first considered for use in biochemical weaponry. A gram of such a toxin is estimated to be capable of killing 200,000 mice. Even more startling, a single cup of this substance in its pure form is estimated to be capable of depopulating the Earth!
A great deal of time was spent during and after the Second World War, by both the Allied and German scientists, to find a method of converting Botulinum Toxin to weapon form. Whilst both of these former enemies have long since forgotten the idea of using these toxins for warfare, concerns still remain over the threat from other organizations, namely terrorists. A chilling example of how such a weapon might be used was shown by the use of a similar reagent, sarin nerve gas, on the Tokyo subway in 1995. 13 people were killed and more than 6000 were injured (50 severe). Some countries are known to have developed Botulinum Toxins in aerosol form, which if used would be far more devastating than Sarin gas.
However, the toxin, up to now, can only have an effect in closed places, as the wind can mix it with air easily, reducing the effectiveness a lot. Nevertheless illegal BTX is still a worry for many nations trying to prevent terrorist groups finding an easy and cheap way of killing people.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5426578]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5426578]