
![]()
1,3-Butadiene
Golf balls, tyres and Lego.
![]()
Paul May
University of Bristol, UK
![]()
Molecule of the Month June 2015
Also available: HTML version.
![]()
|
1,3-ButadieneGolf balls, tyres and Lego.
Paul May
Molecule of the Month June 2015
|
|
They are all made from polymers, from which the main starting material is butadiene.
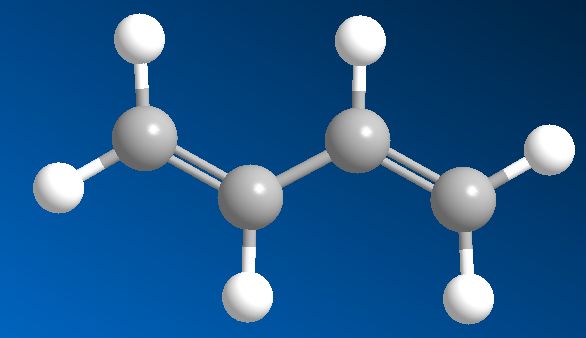
Not really – it’s quite logical. The ‘buta’ part is from butane, meaning 4 carbons. The ‘ene’ suffix means that it contains double-bonded carbons (i.e. it’s an alkene), and the ‘di’ means it has two of them.
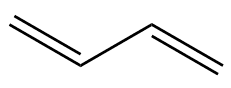
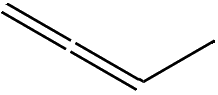
That tells you where the double-bonds are. If you start at one end of the molecule and number the carbon atoms from 1 to 4, the prefix ‘1,3-‘ means that there’s a double-bond starting at carbon-1 (i.e. between C1 and C2), and another starting at carbon-3 (i.e. between C3 and C4). There is also an isomer of butadiene called 1,2-butadiene, but because this has the two double bonds next to each other (a ‘cumulated’ diene) it’s very unstable and so not much use.
 |
 |
1,3-butadiene |
1,2-butadiene |
Because of its double bonds, butadiene can be polymerised into long chains. The Russian chemist Sergei Lebedev (image, right) was the first person to do this in 1910, making one of the first synthetic rubbers.
Two reasons really. If you can synthesise rubber you can change the ingredients and therefore the properties of the rubber to suit different applications. Also, not every country has access to rubber trees. This became particularly important in the middle of the 20th Century, when the various world powers were squaring up for battle in what would eventually become WW2. The British Empire had access to rubber trees in India, while France had them its South East Asia colonies. But Germany had no colonies with rubber trees, nor did Russia, nor did Japan. Nor did the USA, except via its association with the UK and India. And since rubber is an essential component for tyres in most motorised vehicles, wars might be won or lost on whether an army had enough rubber. So an alternative to natural rubber was urgently sought-after by every industrialised country in the world.
 And Lebedev found it?
And Lebedev found it?Yes, in 1928, he developed an industrial method for producing synthetic rubber based on polymerisation of butadiene using metallic sodium as a catalyst, and this was quickly adopted by the Soviets into an industrial process. The butadiene monomer was synthesised from ethanol that the Russians distilled from grain or potatoes, which led to a number of jokes about Russian tyres being made from potatoes! By the time WW2 started the Soviet Union had the largest synthetic rubber industry in the world, and the method had been copied by the Germans, Japanese and Americans allowing those countries, too, to participate in the war.
About 70% of the world’s production of over 2 million tons of polybutadiene per year goes towards making tyres. However, pure polybutadiene is too soft and elastic for most tyres, which need to be harder and more wear resistant. The properties of butadiene can be altered by using different catalysts to change the way it polymerises. The polymer with trans double bonds forms straight rigid rods, which align together making the material behave a bit like a crystal, with a sharp melting point and with stiffer, harder properties.
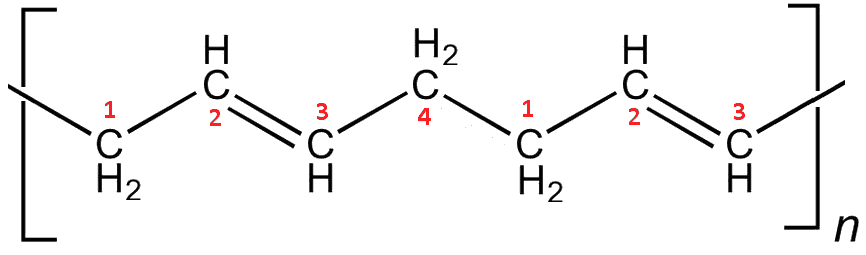
trans-Polybutadiene. The polymerisation occurs between the C1 on one monomer and the C4 on the next, leading to straight chains.
In contrast, the polymer with cis double bonds causes bends in the chain, preventing the chains aligning, resulting in a much more amorphous material. The more cis bonds in the polymer, the more flexible and elastic it becomes.
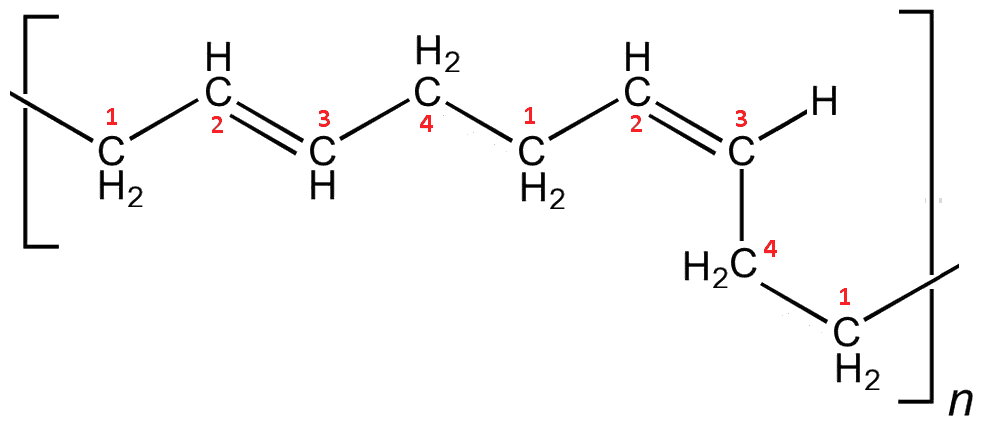
cis-Polybutadiene. This is also a 1-4-polymerisation step, but in the second case the double bond has been rotated into the cis arrangement leading to a bend in the chain.
The ratio of trans to cis double bonds in the synthetic rubber can be controlled by varying the temperature or using a suitable catalyst during the polymerisation process. For example, stiff but hard-wearing high-trans polybutadiene used to be used as the outer layer of golf balls, whereas flexible high-cis polybutadiene is used for tyres.
Yes, the so-called vinyl polybutadienes occur when the polymerisation (using an alkyllithium catalyst) occurs only on the first double bond of a monomer, leaving the remaining C2 group to hang off the chain as a (vinyl) sidegroup. The dangling sidegroups also prevent the chains aligning, so make the polymer more flexible.
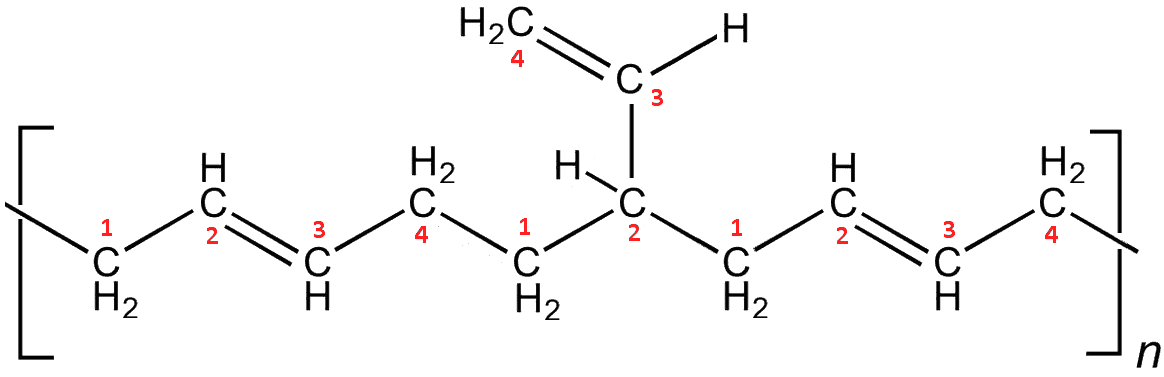
Vinyl-polybutadiene. Here, the second addition has been a 1,2-polymerisation step, with C3 and C4 now dangling off as a vinyl sidegroup. A further 1-4-polymerisation continues the chain.
High-cis is used mainly for tyres, primarily in the sidewalls which need to be flexible. A less flexible rubber would crack from the continual flexing of the tyre as it was in operation. It also helps increase the lifetime of the tyres and fuel efficiency. But it has the disadvantage in that it can be slippery on wet surfaces.
 |
|
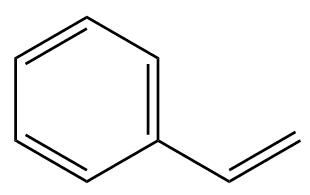
| Styrene |

Tyre manufacturers mix the polybutadiene with another polymer made from butadiene and styrene. So-called styrene-butadiene-rubber (SBR) is used in 50% of car tyres, with the styrene content being used to control the hardness of the rubber.
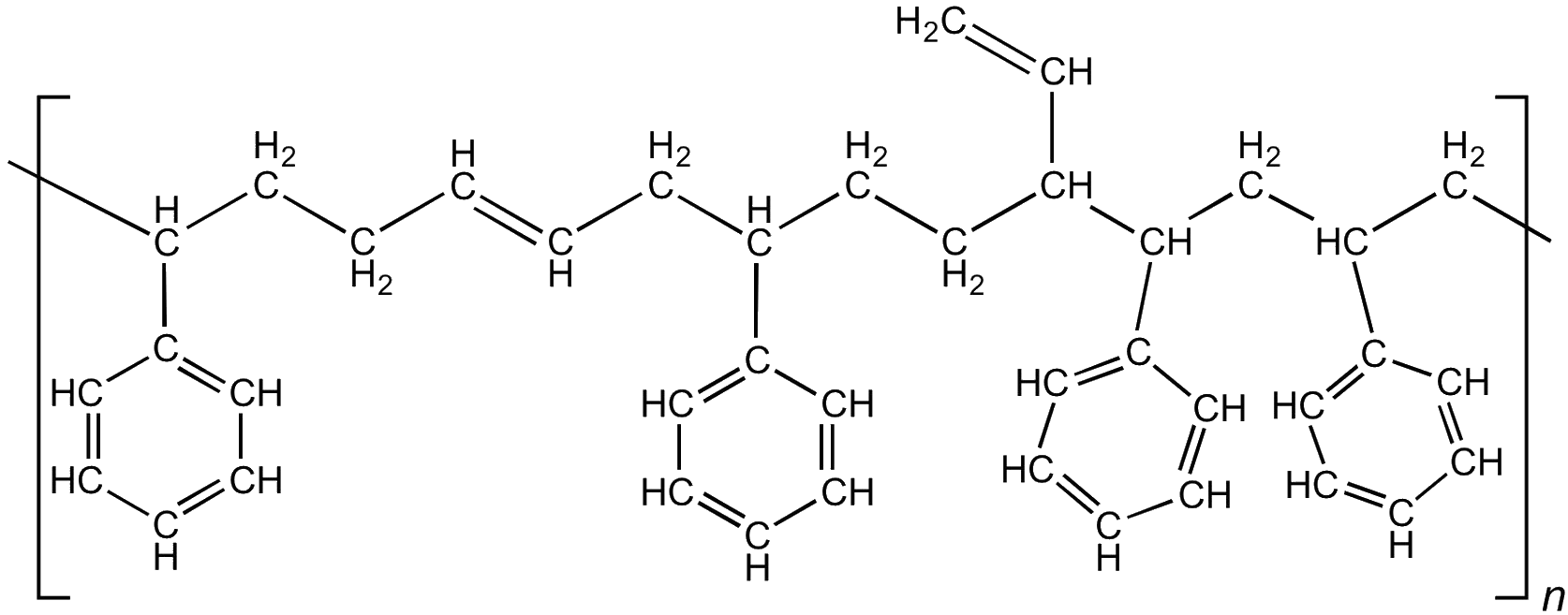
SBR – the polybutadiene backbone can be 1-4 or 1-2 polymerised, giving less or more vinyl groups, respectively.
SBR formed the basis of Ameripol, the synthetic rubber that was used for all US military tyres during WW2. To guarantee rubber supplies, the U.S. government has previously launched a secret initiative to develop synthetic rubber production based on the pre-War development of SBR by Walter Bock in Germany. The result was called GRS (Government Rubber Styrene), although it was just SBR by another name. By 1944, up to 50 US factories were manufacturing GRS at a rate twice that of the world's natural rubber production before the WW2 started.

WW2 trucks with synthetic-rubber tyres.
And speaking of WW2, synthetic rubber was also important for the Germans and Italians, too, and they had their own set of rubber plants which became a strategic target for Allied bombers. Many of these plants were bombed regularly from 1944 onwards as part of the Allied Operation Pointblank aimed at crippling the Axis' production of fighter planes.
Because of its elastic nature, SBR is also used in shoes, gaskets and even chewing gum.
 And Lego bricks?
And Lego bricks?Not quite. Lego is made from another polymer called ABS, whose components are the same as SBR except with another added ingredient, acrylonitrile (CH2=CH-CN). Again, the ratio of the three components changes the properties of the resulting polymer. But ABS usually has high impact resistance and toughness, and is thermoplastic, which means it softens at high temperature but is hard and rigid at lower temperatures. Adding a colouring agent to the mix gives Lego bricks, or computer keyboard keys, or luggage cases. Micron-sized coloured beads of ABS are sometimes used in tattoo inks.
![]()
![]()