The structures of the sites can be better examined with the aid an interactive Chime view of of the 1KCW structure.
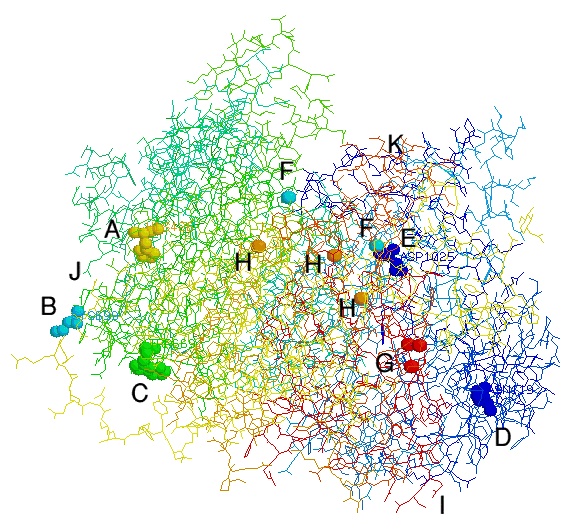
Binding and Active Sites
Proxidant: A
Peroxidase: B
Amine: C
LSD: D
FeII: E
NO: I,J
FeIII: K
| Some progress has been made on the identification
of which regions of the structure have particular functions and which regions
act as binding sites for the various substrates in caeruloplasmin catalysis.
These are described in more detail by Bielli and Calabrese.
The structures of the sites can be better examined with the aid an interactive Chime view of of the 1KCW structure. |
|

|
Binding and Active Sites Proxidant: A Peroxidase: B Amine: C LSD: D FeII: E NO: I,J FeIII: K |
Histidine 426 is described as the proxidant site, responsible for ceruloplasmin's involvement in oxidative damage to proteins. This may be important in LDL (low density lipoprotein) oxidation.
Cysteine 699 is described as the peroxidase active site. This site has also got glutathione peroxidase activity, with hydrogen peroxide and/or organic peroxides.
Para-phenylenediamine is not a natural substrate, but is used in the assay of caeruloplasmin.
This is known to act as a modulator of the oxidase activity of caeruloplasmin to bigenic amines. LSD is of course a psychotomimetic, so does mescalin have a similar course? Caeruloplasmin is found in the brain as a membrane-anchored form.
The ferrous ion can possibly enter here, the region is close to two negatively charged residues anchoring the labile copper atoms. Ferroxidase activity, that is conversion of FeII to FeIII, is probably the most important aspect of caeruloplamin's uses.
Caeruloplasmin may act as a copper transport protein, and the amount of copper is certainly variable. The X-ray structure reveals two extra 'labile' coppers, and many medical researchers refer to caeruloplasmin as having seven coppers. The discovery that copper metabolism is normal in patients with aceruloplasminaemia demonstartes that the metalloprotein has an essential role in iron but not copper metabolism.
This center is responsible in other similar metalloenzymes for the conversion of dioxygen into water. It is important that this feat is accomplished without the release of any of the (nasty!) possible intermediates in this reaction, namely hydrogen peroxide or superoxide radicals.
These are the 'electron transfer' coppers, responsible for the transfer of electrons in and out of the cupredoxins, the one close to the copper cluster is generally thought to ferry electrons into this to aid its reduction of dioxygen.
I, could be an entry point for nitric oxide, J, it's exit in the form of S-nitrosoglutathione. Nitric oxide is a new entry onto the list of substrates for caeruloplasmin.