
 Beta-carotene is the molecule that gives carrots their orange colour. It is part of a family of chemicals called the carotenoids, which are found
in many fruit and vegetables, as well as some animal products such as egg yolks. Carotenoids were first isolated in the early 19th century, and
have been synthesised for use as food colourings since the 1950s.
Beta-carotene is the molecule that gives carrots their orange colour. It is part of a family of chemicals called the carotenoids, which are found
in many fruit and vegetables, as well as some animal products such as egg yolks. Carotenoids were first isolated in the early 19th century, and
have been synthesised for use as food colourings since the 1950s.

 The bright colours found in nature and the molecules which cause them have always fascinated organic chemists. The earliest studies on carotenoids
date back to the beginning of the 19th century. Beta-carotene was first isolated by Wackenroder in 1831, and many other carotenoids were discovered
and named during the 1800s, although their structures were still unknown.
The bright colours found in nature and the molecules which cause them have always fascinated organic chemists. The earliest studies on carotenoids
date back to the beginning of the 19th century. Beta-carotene was first isolated by Wackenroder in 1831, and many other carotenoids were discovered
and named during the 1800s, although their structures were still unknown.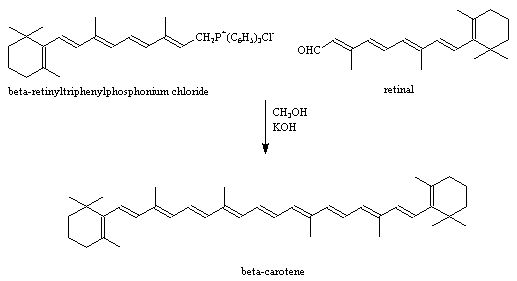

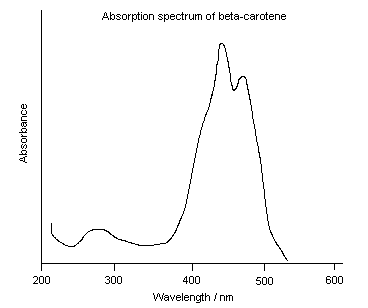

 Natural extracts containing carotenoids, for example carrot extracts and red palm oil, have been used to colour foods for centuries. Synthetic
beta-carotene was first marketed as a food colouring by Roche in 1954. It is mainly used for colouring margarine and butter; its vitamin A activity is an added benefit. Other applications include ice-cream, fruit juice and the coatings of tablets. Beta-carotene has an advantage over other artificial colours, for example azo dyes, because it occurs naturally in food and is so known to be safe.
Natural extracts containing carotenoids, for example carrot extracts and red palm oil, have been used to colour foods for centuries. Synthetic
beta-carotene was first marketed as a food colouring by Roche in 1954. It is mainly used for colouring margarine and butter; its vitamin A activity is an added benefit. Other applications include ice-cream, fruit juice and the coatings of tablets. Beta-carotene has an advantage over other artificial colours, for example azo dyes, because it occurs naturally in food and is so known to be safe. Vitamin A has several functions in the body. The most well known is its role in vision - hence carrots "make you able to see in the dark".
The retinol is oxidised to its aldehyde, retinal, which complexes with a molecule in the eye called opsin. When a photon of light hits the complex,
the retinal changes from the 11-cis form to the all-trans form, initiating a chain of events which results in the transmission of an impulse up the
optic nerve. A more detailed explanation is given here. Other roles of vitamin A are much less well understood. It is known to be involved in the synthesis of certain glycoproteins, and that deficiency leads to abnormal bone development, disorders of the reproductive system, xerophthalmia (a drying condition of the cornea of the eye) and ultimately death.
Vitamin A has several functions in the body. The most well known is its role in vision - hence carrots "make you able to see in the dark".
The retinol is oxidised to its aldehyde, retinal, which complexes with a molecule in the eye called opsin. When a photon of light hits the complex,
the retinal changes from the 11-cis form to the all-trans form, initiating a chain of events which results in the transmission of an impulse up the
optic nerve. A more detailed explanation is given here. Other roles of vitamin A are much less well understood. It is known to be involved in the synthesis of certain glycoproteins, and that deficiency leads to abnormal bone development, disorders of the reproductive system, xerophthalmia (a drying condition of the cornea of the eye) and ultimately death. ![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5346151]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5346151]