
![]()
2-METHYLUNDECANAL
The smell of Chanel No.5
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Molecule of the Month November 2008
Also available: HTML versions.
![]()
 |
2-METHYLUNDECANALThe smell of Chanel No.5
Simon Cotton
Molecule of the Month November 2008
|
"Alone among fragrances known to me, it gives the irresistible impression of a smooth, continuously curved, gold-colored volume that stretches deliciously, like a sleepy panther, from top note to drydown."
Luca Turin
Quatre-vingt ans plus tard, Chanel no.5 resplendit toujours d'un éclat unique, fulgurant.
L'abcdaire du parfum
Gabrielle Bonheur Chanel (1883-1971) had an unpromising start in life. The illegitimate daughter of a pedlar, born at Saumur on the western Loire, her mother died when she was 12. Chanel spent six years in an orphanage at Aubazine in the Corrèze, then 2 years in a boarding school at Moulins, in the Bourbonnais, where she became a dressmaker. It was at Moulins that she acquired the nickname "Coco", from singing a song about a dog with that name. She moved to Paris in 1909 and opened a shop in the boulevard Malesherbes, then a boutique in the rue Cambon, and in 1915 a boutique in Biarritz. There's still a Chanel shop at 31 rue Cambon. By 1920, Chanel's fashions were established. It was time to move into perfumes.
 |

'Coco' Chanel |
 Ernest Beaux (1881-1961) (picture, right) was a Russian-born son of a French father, chief perfumer to A. Rallet & Co., a Moscow soap and perfume manufacturer. Starting as a laboratory technician, Beaux worked his way up to become its technical director and started composing his first perfumes, succeeding with Bouquet de Napoleon (1912). Following the Revolution, he served with the White Russian forces, but left Russia in 1919 to settle near Cannes, at Rallet's new factory at La Bocca, close to the perfume capital of Grasse. He was introduced to Coco Chanel by her lover, Grand Duke Dimitri Pavolivch, another Russian émigré - on the beach at Cannes, according to some accounts. Chanel is said to have asked Beaux to create a scent that "reflects my personality, something abstract and unique." According to one source, Beaux believed that he had created something that reminded him of snow-covered Russian landscapes. At all events, Chanel No.5 was a real coup de Grasse.
Ernest Beaux (1881-1961) (picture, right) was a Russian-born son of a French father, chief perfumer to A. Rallet & Co., a Moscow soap and perfume manufacturer. Starting as a laboratory technician, Beaux worked his way up to become its technical director and started composing his first perfumes, succeeding with Bouquet de Napoleon (1912). Following the Revolution, he served with the White Russian forces, but left Russia in 1919 to settle near Cannes, at Rallet's new factory at La Bocca, close to the perfume capital of Grasse. He was introduced to Coco Chanel by her lover, Grand Duke Dimitri Pavolivch, another Russian émigré - on the beach at Cannes, according to some accounts. Chanel is said to have asked Beaux to create a scent that "reflects my personality, something abstract and unique." According to one source, Beaux believed that he had created something that reminded him of snow-covered Russian landscapes. At all events, Chanel No.5 was a real coup de Grasse.
 The perfume
The perfumeWe still think think of perfumes being made of extracts from flowers, and have visions of Provencal lavender fields, and of perfumers in Grasse steam-distilling roses to extract essential oils. Well, that age of entirely "natural" perfumes vanished in the 1880s, with the use of synthetic coumarin in Fougère Royale (1882) and of synthetic vanillin in Jicky (1887; still on sale today). So Chanel No.5 wasn't the first to use synthetics, but it was one of the first to make major use of aldehydes.
In 1912, the perfumer Robert Bienaimé first used 2-methylundecanal in the successful perfume Quelques Fleurs for Houbigant. Ernest Beaux imitated this breakthrough in his new perfume Rallet No. 1 (1914). Recent analysis using original sealed bottles of Rallet No. 1 has established his use of undecanal, dodecanal and undec-10-enal, along with neroli and ylang-ylang, for the top note; jasmine absolute, rose de mai, orris and lily of the valley for the middle note; and sandalwood, vetiver, styrax, vanillin and nitromusk in the base note.
There are all sorts of stories about the creation of Chanel No.5. It is said that Beaux gave Coco several labelled bottles to choose from, and she chose No.5, as her lucky number. Others say that Beaux's assistant added too much aldehyde, and that the resulting perfume was a piece of luck.
All perfumes are mixtures of chemicals, and can be viewed in terms of three components.
 Top note (head note), the initial impact of a fragrance, which predominates for the first few minutes, leading to...
Top note (head note), the initial impact of a fragrance, which predominates for the first few minutes, leading to...
Middle note (heart note), which contains the main fragrances and personality of the perfume, and lasts for several hours. Eventually this is replaced by...
Bottom note (base note, end note or drydown), is the residual smell, which persists when all else has fled, and remains with you until the perfume has totally evaporated. These molecules also help to reduce the volatility of the others.
When he composed No. 5, Beaux used a mixture for each note. Today they are, for the top note, ylang-ylang (i.e. floral), neroli (spicy sweet floral) and aldehydes with 10-12 carbons; for the middle, a mixture of jasmine, rose, lily of the valley and iris; and for the base note sandalwood, vetiver, musk, vanilla, civet and oak moss. Not too dissimilar to his Rallet No. 1, with the important addition of 2-methylalkanals, especially 2-methylundecanal.
The odours of aliphatic aldehydes vary strongly with the size and shape of the molecules. Short-chain aldehydes have rather sharp and unpleasant odours (just think of ethanal, with its fruity and pungent smell); these become milder as the carbon chain lengthens. From C8-C12, the odours have a waxy-floral note, the odours becoming faint by C14.
|
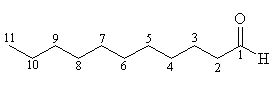
The carbon numbering of an aldehyde, with n=11 (undecanal). |
Introducing a methyl side chain at carbon 2 makes the odour stronger and also more pleasant, reducing the fatty note.
|
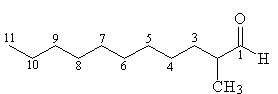
2-substituted aldehyde with n=11 |
If the side chain has more than two carbons, however, the odour becomes progressively weaker.
Which brings us to 2-methylundecanal...
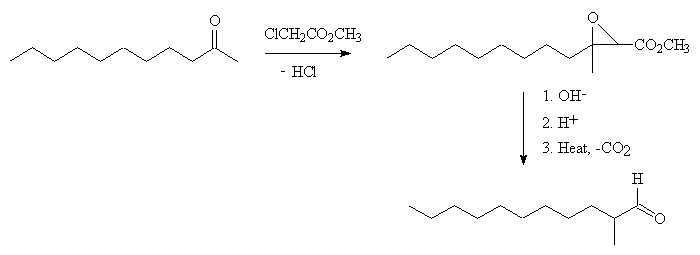
A convenient synthesis of 2-methylundecanal starts with 2-undecanone; this reacts with methylchloroacetate in the presence of the base sodium tert-butoxide, producing a glycidyl ester in a Darzens condensation reaction. This can be hydrolysed by refluxing with alkali, then acidified to convert it into the corresponding acid. This decarboxylates on distillation under reduced pressure, forming 2-methylundecanal.
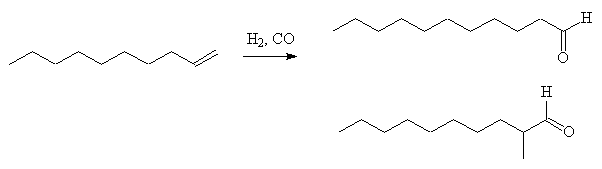
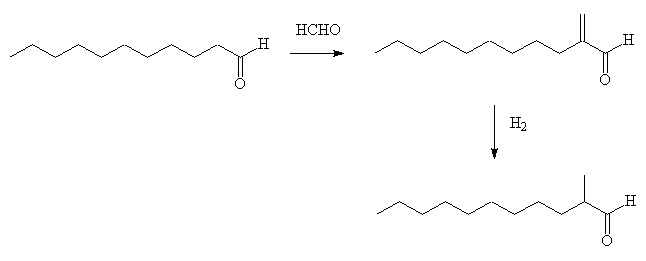
In another approach, dec-1-ene is hydroformylated to produce a mixture of mainly undecanal and 2-methyldecanal. The undecanal reacts with formaldehyde in the presence of dibutylamine giving 2-methylene undecanal in high yield; this can be hydrogenated and the 2-methylundecanal be obtained pure by fractional distillation of the mixture.
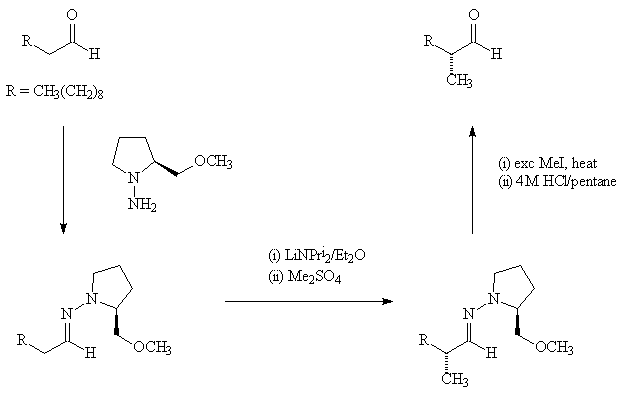
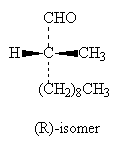 2-methylundecanal has a chiral carbon, and synthesis of the two enantiomers has been achieved, starting from the straight chain molecule undecanal. This makes use of the chiral hydrazone molecules (R)-(+)-1-amino-2-(methoxymethyl)pyrrolidine (RAMP) and (S)-(-)-1-amino-2-(methoxymethyl)pyrrolidine (SAMP), obtainable as the pure enantiomers, to prepare the separate aldehyde hydrazones. These are metallated with lithium diisopropylamide and alkylated with dimethyl sulphate to introduce the sidechain; the resulting hydrazone can be cleaved at the C=N bond and converted into the aldehyde.
2-methylundecanal has a chiral carbon, and synthesis of the two enantiomers has been achieved, starting from the straight chain molecule undecanal. This makes use of the chiral hydrazone molecules (R)-(+)-1-amino-2-(methoxymethyl)pyrrolidine (RAMP) and (S)-(-)-1-amino-2-(methoxymethyl)pyrrolidine (SAMP), obtainable as the pure enantiomers, to prepare the separate aldehyde hydrazones. These are metallated with lithium diisopropylamide and alkylated with dimethyl sulphate to introduce the sidechain; the resulting hydrazone can be cleaved at the C=N bond and converted into the aldehyde.
The scheme shows the synthesis of (R)-(-)-2-methylundecanal using (S)-SAMP. The two isomers have very similar smells.
 The bottle...
The bottle...The Chanel No. 5 bottle is iconic in itself. In contrast to the ornate, complex and romantic bottles fashionable at the time, Chanel chose something purely functional - and also cheap to manufacture. Its design is said to be based on a male cologne bottle, possibly that used by the great love of her life, Arthur "Boy" Capel, who was killed in a car crash on December 24th 1919. Its design has not remained constant, small and subtle changes are made every 20 years or so. The bottle and its packaging are an essay in minimalism.
Andy Warhol was later to make a series of silkscreen prints of the bottle.
Initially, Chanel promoted the perfume herself, giving bottles to favoured clients. The marketing of Chanel No. 5 only took off in 1924 after she had done a deal with Pierre Wertheimer, owner of the Bourjois cosmetics and fragrance company; Coco Chanel had her own couture business, but only took 10% of the profits from the perfumes, something which was to rankle with her for years.
Ever since Marilyn Monroe's remark that she only slept wearing a few drops of No. 5, a host of beautiful and famous women have been associated with its advertising.



Marilyn Monroe, Ali McGraw and Catherine Deneuve in posters advertising Chanel No.5.


Carole Bouquet, Nicole Kidman and Audrey Tautou also advertising Chanel No.5.
"One has to rely on chemists to find new aroma chemicals creating new, original notes. In perfumery, the future lies primarily in the hands of the chemists."
Ernest Beaux
![]()
Acknowledgements: I am most grateful to Charles Sell and to James Anderson for helpful information.
![]()