 |
|
| Chlorhexidine |
|
 It is a must have ingredient for mouthwashes, I’ve noticed...
It is a must have ingredient for mouthwashes, I’ve noticed...
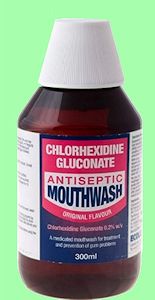
Yes, that’s because chlorhexidine is a bactericide, and is also effective against some fungi and viruses (although less so). The form used in mouthwashes is 0.2% chlorhexidine gluconate. It is not present in the most popular mouthwashes you would find in supermarkets, but rather in the more specialised ones that your dentist will prescribe, for instance when you have gingivitis (swelling of the gums) or inflammation when your wisdom teeth are coming up. Having said that, mouthwashes and gels containing chlorhexidine are sold over the counter in the UK (but not in the US, where a prescription is needed).
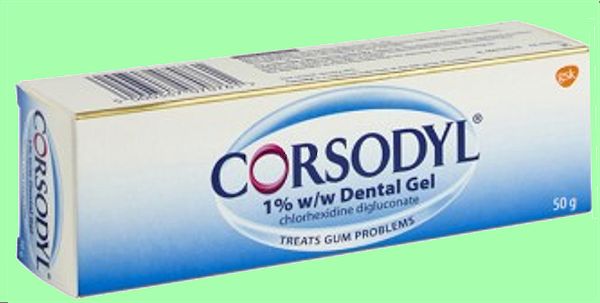
They are often prescribed before, during or after root canal or gum disease treatment to keep infection at bay.
And new ingenious ways of using chlorhexidine for dental treatments continue to be developed, for instance, chlorhexidine-containing nanoparticles for root canal disinfection have been formulated.
There are many bactericidal substances, why is this one in particular used in mouthwashes?
Well, its toxicity is low, and it is mostly non-irritant. But a trick chlorhexidine has up its sleeve is its ability to bind to oral tissues and to be released slowly into the oral cavity, or mouth, to you and me. This property is known as 'substantivity'.
It allows chlorhexidine to provide a continued inhibitory effect on plaque formation for up to 14 hours.
The effectiveness of chlorhexidine is documented in many controlled clinical trials showing a 50% to 60% decrease in plaque, a 30% to 45% reduction in gingivitis, and a reduction in the number of oral bacteria.
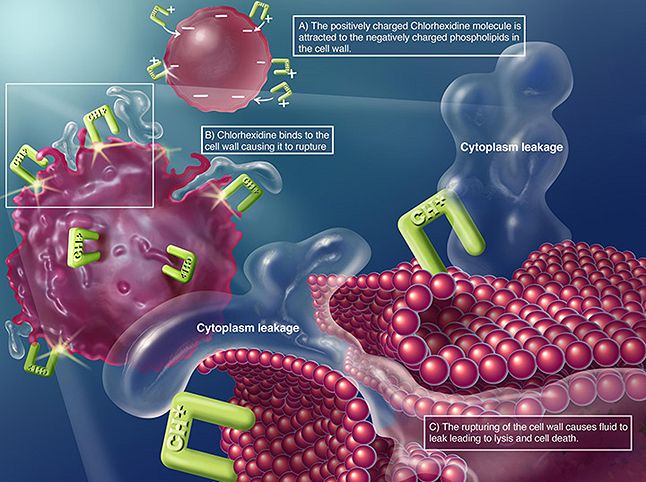
Antibacterial mechanism of chlorhexidine.
[Image from: chlorhexidinefacts.com]
But how does it work?
Chlorhexidine affects the bacterial cell wall. And then, when the cell wall is damaged, chlorhexidine can permeate it and attack the cell membrane. This produces leakage of cytoplasmic material leading to cell death. Used in certain concentrations, it can also cause the cytoplasmatic fluid to precipitate.
 Could it replace the toothbrush?
Could it replace the toothbrush?
That would be nice, but no. Chlorhexidine doesn’t tackle tartar build up, so we still need to follow a regular programme of dental hygiene that includes twice-daily tooth brushing, daily flossing and regular visits to your dentist.
It’s an antiseptic, so surely it has other uses?
Yes, it is used in many topical antiseptic and bactericidal products: handwashes, soaps, sprays, gels, creams and skin-wipes. It is impregnated into some wound-dressings, catheters and surgical equipment. It is also present in some pet-shampoos and is used widely to treat bacterial skin infections in dogs and cats.
A remarkable life-saving use is umbilical cord stump care, and Nepal was the first country to introduce chlorhexidine gel for this. About 70% of under-five deaths in the country occur within the first month of life. Of these, around 50% are down to infection. The treatment of the stump with chlorhexidine has decreased neonatal deaths by 24% and neonatal infections by 68%.
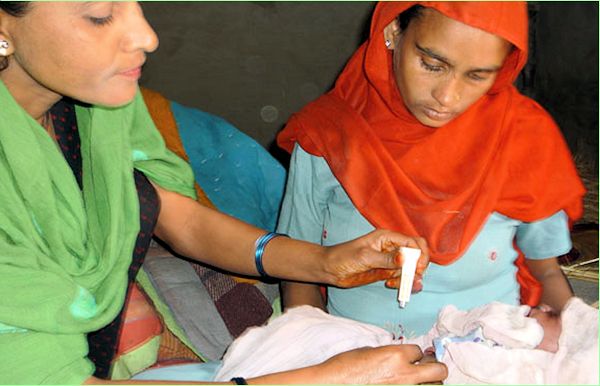
A community health volunteer applies chlorhexidine to the
umbilical cord of a newborn delivered at home in the Banke district.
[Image: Nima Gupta, Nepal Family Health Program]
Chlorhexidine is on the WHO's List of Essential Medicines and since 2013, the organisation recommends the use of 7.1% chlorhexidine gel or solution to treat umbilical cord stumps immediately after cutting to prevent infection (a condition known as omphalitis of the newborn).

Products containing chlorhexidine.
What about side effects?
Chlorhexidine mouthwash can cause brown stains on teeth because its substantivity means it can bind to hard as well as soft tissues. And this is the main reason why it isn’t in toothpaste, although attempts have been made. People who regularly use chlorhexidine wash, are advised not to drink too much coffee, tea, red wine or consume foods that may be likely to stain their teeth.
It can also cause painful peeling – called desquamation – of the outer layer of the mouth’s mucosa, but this effect is rare. Some people find it has an unpleasant taste or changes the taste of some foods, which can undermine compliance with the treatment.
Chlorhexidine can also contribute to the accumulation of the chalky substance in our teeth known as tartar.
Allergic reactions or hypersensitivity are rare but can be serious. Patch testing of chlorhexidine has revealed positive reactions in around 2% of patients tested and up to 5% in patients with eczema. And a small but significant number of cases of anaphylaxis have been reported. So, research is being carried out into alternatives.
These side-effects also explain why chlorhexidine is not just put into toothpaste.
Is there anyone who cannot use it?
Yes, apart from those allergic to it, chlorhexidine mouthwashes may not be the best choice for people with tooth crowns or caps made of composite or glass ionomer, as these materials may stain.
Being a bactericide, have resistance problems emerged?
Yes, there are some concerns, and research is being carried out into this. Resistance to chlorhexidine in dental bacteria has not yet been reported as a major issue. But a worrying discovery is that some bacteria can develop resistance to other substances when exposed to chlorhexidine. For instance, Klebsiella pneumoniae can become resistant to colistin, a last resort antibiotic, often used against multidrug resistant pathogens, when exposed to chlorhexidine-based disinfectants.
And what is the origin of chlorhexidine?
It was discovered in the 1950s by the Imperial Chemical Industries, Ltd (Manchester, UK) and it was introduced as a disinfectant and topical antiseptic in 1954. It was first used in handwashes in 1970, having been shown to reduce the skin flora by around 90%. It was then tested as an oral agent in 1976 and demonstrated the ability to inhibit the formation and development of plaque.
And how was it discovered?
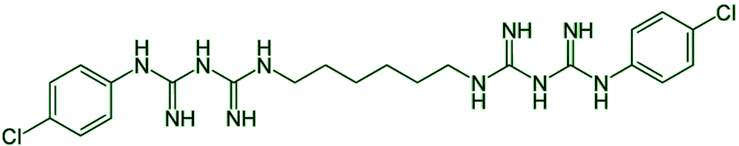
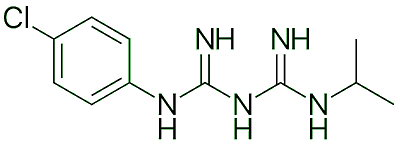
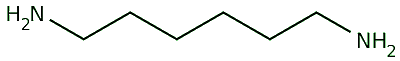
It was discovered while researching the synthesis of anti-malarial agents. The structure is based on two molecules of Proguanil (a known antimalarial drug), linked with a hexamethylenediamine spacer. But other synthesis routes have been used, such as combining hexamethylene-bis-dicyanodiamide and 4-chloroaniline hydrochloride.
|
 |
 |
|
|
Proguanil |
Hexamethylenediamine |
|
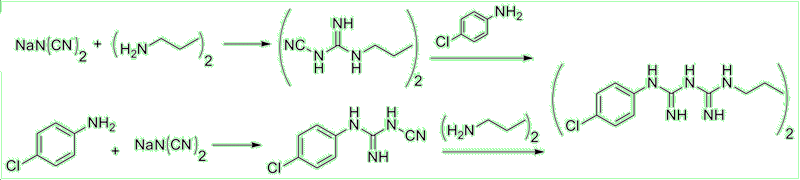
Two synthetic routes to chlorhexidine.

Bibliography
Overview and mechanism
Dental uses
Neonatal use
Side effects and resistance


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12442520]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12442520]
![]()
![]()
![]()
![]()


 It is a must have ingredient for mouthwashes, I’ve noticed...
It is a must have ingredient for mouthwashes, I’ve noticed...

 Could it replace the toothbrush?
Could it replace the toothbrush?




