![]()

 Chlorophyll
Chlorophyll

![]()
Paul May
School of Chemistry, University of Bristol
![]()
VRML, Jmol, and Chime versions
Chlorophyll is the molecule that absorbs sunlight and uses its energy to synthesise carbohydrates from CO2 and water. This process is known as photosynthesis and is the basis for sustaining the life processes of all plants. Since animals and humans obtain their food supply by eating plants, photosynthesis can be said to be the source of our life also.

| Chlorophyll is the green coloration in leaves. |
 Photosynthesis
PhotosynthesisIn 1780, the famous English chemist Joseph Priestley (right) found that plants could "restore air which has been injured by the burning of candles." He used a mint plant, and placed it into an upturned glass jar in a vessel of water for several days. He then found that "the air would neither extinguish a candle, nor was it all inconvenient to a mouse which I put into it". In other words, he discovered that plants produce oxygen.
 A few years later, in 1794, the French chemist Antoine Lavoisier (left), discovered the concept of oxidation, but soon after was executed during the French Revolution for being a Monarchist sympathiser. The judge who pronounced sentence said "The Republic has no need for scientists".
A few years later, in 1794, the French chemist Antoine Lavoisier (left), discovered the concept of oxidation, but soon after was executed during the French Revolution for being a Monarchist sympathiser. The judge who pronounced sentence said "The Republic has no need for scientists".
 So it fell to a Dutchman, Jan Ingenhousz (left), who was court physician to the Austrian empress, to make the next major contribution to the mechanism of photosynthesis. He had heard of Priestley's experiments, and a few years later spent a summer near London doing over 500 experiments, in which he discovered that light plays a major role in photosynthesis.
So it fell to a Dutchman, Jan Ingenhousz (left), who was court physician to the Austrian empress, to make the next major contribution to the mechanism of photosynthesis. He had heard of Priestley's experiments, and a few years later spent a summer near London doing over 500 experiments, in which he discovered that light plays a major role in photosynthesis.
"I observed that plants not only have the faculty to correct bad air in six to ten days, by growing in it...but that they perform this important office in a complete manner in a few hours; that this wonderful operation is by no means owing to the vegetation of the plant, but to the influence of light of the sun upon the plant".
 Very soon after, more pieces of the puzzle were found by two chemists working in Geneva. Jean Senebier, a swiss pastor, found that "fixed air" (CO2) was taken up during photosynthesis, and Theodore de Saussure discovered that the other reactant necessary was water. The final contribution to the story came from a German surgeon, Julius Robert Mayer (right), who recognised that plants convert solar energy into chemical energy. He said:
Very soon after, more pieces of the puzzle were found by two chemists working in Geneva. Jean Senebier, a swiss pastor, found that "fixed air" (CO2) was taken up during photosynthesis, and Theodore de Saussure discovered that the other reactant necessary was water. The final contribution to the story came from a German surgeon, Julius Robert Mayer (right), who recognised that plants convert solar energy into chemical energy. He said:
"Nature has put itself the problem of how to catch in flight light streaming to the Earth and to store the most elusive of all powers in rigid form. The plants take in one form of power, light; and produce another power, chemical difference."
The actual chemical equation which takes place is the reaction between carbon dioxide and water, powered by sunlight, to produce glucose and a waste product, oxygen. The glucose sugar is either directly used as an energy source by the plant for metabolism or growth, or is polymerised to form starch, so it can be stored until needed. The waste oxygen is excreted into the atmosphere, where it is made use of by plants and animals for respiration.
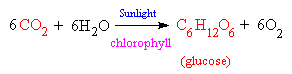
Chlorophyll is the molecule that traps this 'most elusive of all powers' - and is called a photoreceptor. It is found in the chloroplasts of green plants, and is what makes green plants, green. The basic structure of a chlorophyll molecule is a porphyrin ring, co-ordinated to a central atom. This is very similar in structure to the heme group found in hemoglobin, except that in heme the central atom is iron, whereas in chlorophyll it is magnesium.
There are actually 2 main types of chlorophyll, named a and b. They differ only slightly, in the composition of a sidechain (in a it is -CH3, in b it is CHO). Both of these two chlorophylls are very effective photoreceptors because they contain a network of alternating single and double bonds, and the orbitals can delocalise stabilising the structure. Such delocalised polyenes have very strong absorption bands in the visible regions of the spectrum, allowing the plant to absorb the energy from sunlight.
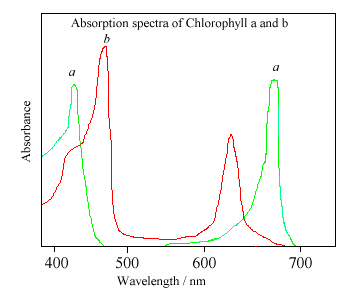
The different sidegroups in the 2 chlorophylls 'tune' the absorption spectrum to slightly different wavelengths, so that light that is not significantly absorbed by chlorophyll a, at, say, 460nm, will instead be captured by chlorophyll b, which absorbs strongly at that wavelength. Thus these two kinds of chlorophyll complement each other in absorbing sunlight. Plants can obtain all their energy requirements from the blue and red parts of the spectrum, however, there is still a large spectral region, between 500-600nm, where very little light is absorbed. This light is in the green region of the spectrum, and since it is reflected, this is the reason plants appear green. Chlorophyll absorbs so strongly that it can mask other less intense colours. Some of these more delicate colours (from molecules such as carotene and quercetin) are revealed when the chlorophyll molecule decays in the Autumn, and the woodlands turn red, orange, and golden brown. Chlorophyll can also be damaged when vegetation is cooked, since the central Mg atom is replaced by hydrogen ions. This affects the energy levels within the molecule, causing its absorbance spectrum to alter. Thus cooked leaves change colour - often becoming a paler, insipid yellowy green.
 | As the chlorophyll in leaves decays in the autumn, the green colour fades and is replaced by the oranges and reds of carotenoids. |
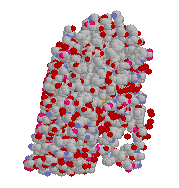 The chlorophyll molecule is the active part that absorbs the sunlight, but just as with hemoglobin, in order to do its job (synthesising carbohydrates) it needs to be attached to the backbone of a very complicated protein. This protein may look haphazard in design, but it has exactly the correct structure to orient the chlorophyll molecules in the optimal position to enable them to react with nearby CO2 and H2O molecules in a very efficient manner. Several chlorophyll molecules are lurking inside this bacterial photoreceptor protein (right).
The chlorophyll molecule is the active part that absorbs the sunlight, but just as with hemoglobin, in order to do its job (synthesising carbohydrates) it needs to be attached to the backbone of a very complicated protein. This protein may look haphazard in design, but it has exactly the correct structure to orient the chlorophyll molecules in the optimal position to enable them to react with nearby CO2 and H2O molecules in a very efficient manner. Several chlorophyll molecules are lurking inside this bacterial photoreceptor protein (right).
