Cisplatin and Chemical Software
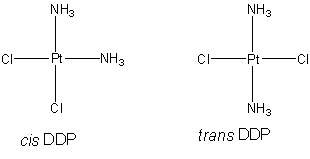
Although some students of organic chemistry may be accustomed to interpreting a
tetracoordinate atom as a flattened tetrahedron, cisplatin has a square planar
geometry. This shape minimizes repulsive interactions between electrons in
the d orbitals.
There is a difference between cis and
trans forms of a complex with two sets of identical ligands about
a central, planar atom.
Therefore, cisplatin provides a useful
shibboleth when examining a chemical information software package. Most such
packages recognize stereochemistry about tetrahedral carbon, but few
can discriminate between the cis and trans isomers of planar
diamminedichloroplatinum(II).
Try an exact-match search on cisplatin using your software and see if you also
hit the trans isomer!
Please see the reference frame for a list of suggestions on learning more
about the factors that affect the geometry of metal complexes.

