![]()
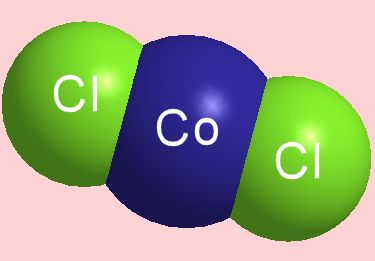
COBALT CHLORIDE
A drug used to dope racehorses that's also a water indicator.
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month June 2016
Also available: HTML version.
![]()
 |
COBALT CHLORIDEA drug used to dope racehorses that's also a water indicator.
Simon Cotton
Molecule of the Month June 2016
|
Why?
True, but this picture shows the structure of an isolated CoCl2 molecule in the gas phase at around 1000 K. Electron diffraction measurements show that it is a linear molecule with Co-Cl distance of 2.113 Å. You can also obtain isolated CoCl2 molecules in matrices of argon and nitrogen at low temperatures too (the unreactive Ar atoms and N2 molecules come between CoCl2 molecules, ‘spacing them out’).
It is an unusual material in the way it changes colour, particularly in the presence of water. Anhydrous cobalt chloride, CoCl2, is blue in colour. As it absorbs water, it turns pink.
Precisely.
Well, its structure rearranges. First the purple dihydrate, CoCl2.2H2O, forms and then at higher humidity the pink hexahydrate CoCl2.6H2O is the product. You can reverse these changes with solid cobalt chloride crystals. If you warm pink CoCl2.6H2O gently – up to 150°C or so - it will gradually lose water forming the violet CoCl2.2H2O, then blue anhydrous CoCl2. This can again be reversed by adding water.
| CoCl2 (s) |  |
CoCl2.2H2O (s) |  |
CoCl2.6H2O (s) |
| blue | purple | pink |
You can get an indicator paper – like litmus paper but containing cobalt chloride - which changes colour from blue to pink in the presence of water.
 |
 |
Cobalt chloride 'water indicator' paper. |
Self-indicating silica gel. |
It can be used as a humidity indicator in weather-measuring instruments, as well as in self-indicating silica gel.
Yes, they are put in with various consumer products, like electrical goods, shoes and handbags, to absorb water, because silica gel can absorb up to about 40% of its weight in water. When you heat the gel, it loses the water so you can reuse it. Silica gel is sometimes supplied containing some cobalt chloride, which indicates the water content – blue when it is dry, pale pink when the air round it is damp.
Anhydrous CoCl2 has the same structure as cadmium chloride, CdCl2. Each cobalt is surrounded by six chlorides, each chloride being shared between three cobalts.
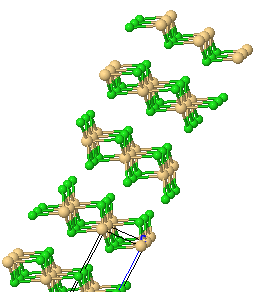 |
-chloride-layer-3D-balls.gif) |
A part of the CoCl2 lattice. |
A smaller section of a Cobalt(II)-chloride-layer. |
CoCl2.2H2O has a chain structure in which each cobalt atom is bound to 4 bridging chlorides and to two water molecules.
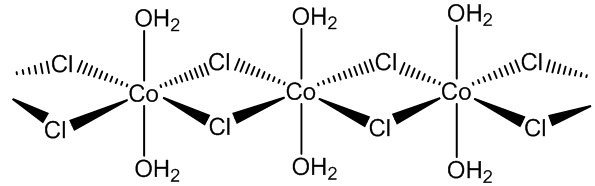
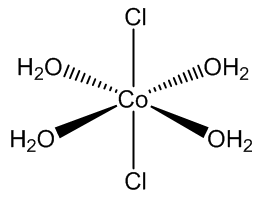
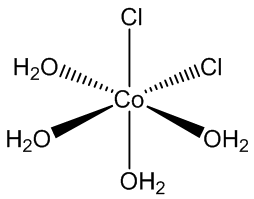
CoCl2.6H2O contains trans-[CoCl2(H2O)4] molecules, with two molecules of ‘water of crystallisation’ in the lattice, so you can write it [CoCl2(H2O)4].2H2O. If you carry out the crystallisation of cobalt chloride around 50ºC, you get the tetrahydrate CoCl2.4H2O, which surprisingly contains cis-[CoCl2(H2O)4] molecules.
 |
 |
trans-[CoCl2(H2O)4] molecules |
cis-[CoCl2(H2O)4] molecules |
Yes, normally the bulky chloride ligands stay as far apart as possible in the coordination sphere. In the tetrahydrate CoCl2.4H2O, it appears that hydrogen bonds between chlorides and the hydrogen atoms in water molecules in the next CoCl2(H2O)4 molecule along make the cis structure more favourable. It shows that it is the balance of small things that can tip the balance and determine a structure.
It looks as if [Co(H2O)6]2+ and [Co(H2O)5Cl]+ ions are the main species present in solutions of CoCl2.
Quite a few tetrahedrally coordinated Co(II) complexes, like those containing the [CoCl4]2- ions, are blue in colour, so at one time people believed it was because anhydrous CoCl2 contained tetrahedrally coordinated Co(II). We now know that this is not the case.
It can be explained using ligand-field theory. The colour of transition metal complexes is due to electronic transitions between different d-orbitals. Water produces larger splittings between the energy levels than chloride (H2O is a ‘stronger-field’ ligand than Cl) so the energy differences between different electronic energy levels are smaller in complexes with lots of chlorides as ligands, so this has a significant effect on the colour.
Most often they do, but the coordination number of the metal is largely dictated by how many donor atoms can fit around the central metal. The later transition metals are smaller than the earlier ones - on crossing the 3d transition series, the nuclear charge (and atomic number) increase, but the screening due to filled electronic shells stay the same. This draws the outer electrons in closer, so the atoms (and ions) get progressively smaller. For zinc, the last – and smallest - of the 3d metals, four is probably the most common coordination number.
Quite a lot of study has been made of complexes of cobalt chloride with pyridine-type ligands. Pyridine forms more than one complex with CoCl2. The pink 4:1 complex has a trans-octahedral arrangement, [CoCl2(pyridine)4].
4.gif) |
|
CoCl2(py)4 | |
Co(py)2Cl2 exists in pink (α) and blue (β) forms, the pink one being more stable at room temperature; the pink form changes into the blue form at 120ºC, but this blue form reverts to pink on standing at room temperature. The pink form has a polymeric structure, with octahedral coordination of cobalt, whilst the blue form has a tetrahedral structure.
2Cl2.gif) |
2Cl2.gif) |
pink Co(py)2Cl2 |
blue Co(py)2Cl2 |
As substituents are introduced into the pyridine rings, so the ligands get bulkier, there is space for fewer ligands around the cobalt, and thus the tetrahedral form becomes more stable, as found in CoCl2(3-methylpyridine)2, CoCl2(4-methylpyridine)2 and CoCl2(3,5-dimethylpyridine)2.
Yes, complexes with tertiary phosphines and phosphine oxides, like CoCl2(PPh3)2 and CoCl2(OPPh3)2 also have tetrahedral structures. Mind you, using the terdentate ligand terpyridyl (2,2';6',2"-terpyridine) makes a five-coordinate complex, Co(terpy)Cl2, possible.
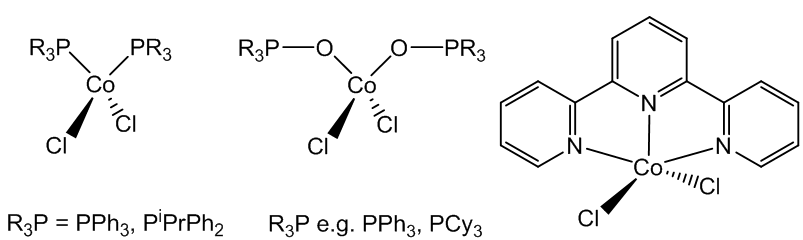
(Left) Tetrahedral Co complexes with tertiary phosphines and phosphine oxides, and (right) Co(terpy)Cl2
 Is there any more to cobalt chloride than coordination chemistry?
Is there any more to cobalt chloride than coordination chemistry?It has attracted quite a bit of attention as a doping agent in sport, especially in horse racing. It has a similar effect to another notorious doping agent, erythropoietin (EPO), in that it stimulates the production of red blood cells, so the blood can carry more oxygen. In the 1990s recombinant EPO became very widely used by professional cyclists in particular, as obviously if you increase your red cell count you improve your oxygen-carrying capacity and improve performance - so it is strongly implicated in blood-doping. As with EPO, there is a downside to using cobalt chloride for doping, as the greater number of red cells makes the blood more viscous and there is an increased risk of heart attacks (‘Regular intake of high cobalt salt doses comes with a real risk of organ injury such as thyroid dysfunction, cardiotoxicity, and heart failure’). Nevertheless, it is an attractive agent to cheats as there is only a short detection window (4 to 6 hours). Recently, several Australian horse trainers have been under investigation for this type of doping; three trainers and a vet have received bans, one of 15 years.
![]()
![]()