
[Image: JialiangGao, CC BY-SA 4.0 via Wikimedia Commons]
|

Curare
(D-Tubocurarine)
The South American dart poison known
as the 'Flying death'

Sophie Goodall
University of Bristol

Molecule of the Month - August 2024
Also available: JSMol version.

|

Bullseye! |
What do you mean by dart poison?
Indigenous people in Central and South America smear curare as a paste onto the tips of darts which are then shot from a blowpipe at birds and small animals for hunting. Once struck by the dart, the paralytic poison enters into the animal's bloodstream, and the prey animals die within a few minutes due to asphyxiation from the animal’s inability to contract their respiratory muscles.
It's an interesting name for a molecule.
Curare is actually the name given to a mixture of toxins, the main one of which is D-tubocurarine. But the name curare originates from the native Guyana Mukusi Indian word ourari, which means 'arrow poison'. The name was based on the word uirarei which was a changed form of the original word uria, meaning 'bird' and eor meaning 'kill'.
So, there are different curare names?
Yes, there are different names because curare is in fact the general term for different types of preparations, made from different plants and which contain different toxins. German pharmacologist Rudolf Boehm differentiated the types of curare preparations based on the type of container in which they were stored. D-Tubocurarine is so-called because on transportation from South America to Europe, the plant extract was packed in bamboo tubes. Similarly, curare which was stored in calabash gourds were called 'calabash curare', and those packed in small earthenware pots were called 'pot curare'. However, the classification of curares into the three categories "calabash", "tube", and "pot" became invalid shortly after they were first coined, because it was subsequently found that there were actually no rules about which containers stored different curares!
 |
 |
South American Indians preparing an arrow poison of curare.
[Image: Wellcome Image file, CC BY 4.0 via Wikimedia Commons] |
Alexander von Humboldt in 1843.
[Image: Joseph Karl Stieler, Public domain, via Wikimedia Commons ] |
So, how exactly is it produced?
The German explorer and naturalist Alexander von Humboldt provided the first reliable eye-witness account of
curare preparation in 1832. He noted that the Ticunas people prepared curare in the rain forests of the Amazon and Orinoco from crude dried extracts of the stems and barks of numerous plants. The method was to collect young bark scrapings, pound them, and boil the fibrous mass in water for 48 hours. The liquid was then strained and evaporated to create a dark, heavy, sticky paste, with a bitter taste. Other plant additives were used as it helped with the preparation and made the poison tacky so it would stick to the arrow or darts. Animal products such as the fangs of venomous snakes or venomous ants and spiders were added to increase the usefulness of the poison.
  |
 |
Left: Chondrodendron tomentosum, is a climbing plant and the main source of D-tubocurarine.
Right: close-up of the vine structure from which the drug is extracted.
|
Strychnos toxifera (a.k.a. bush rope or devil doer)
whose main active constituent is the alkaloid toxiferine.
[Image: Reinaldo aguilar, CC BY-NC-SA 2.0 DEED licence via Flikr] |
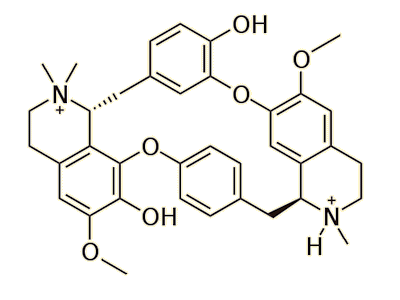
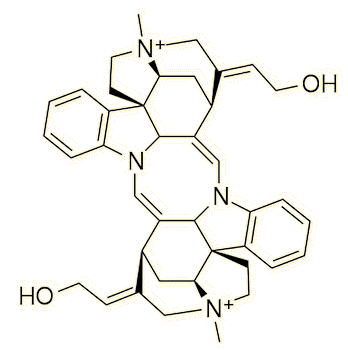
We now know that there are two main types of curare preparation; those derived from Chondrodendron or other members of the Menispermaceae family have D-tubocurarine as the main active ingredient, while those derived from Strychnos toxifera have the alkaloid toxiferine as the main active constituent. There are other preparations which contain a mixture from both curares plus a number of secondary ingredients.
 |
 |
| D-tubocurarine |
Toxiferine |
How did indigenous people assess the potency of the arrow poison?
Curare is only toxic if it enters the bloodsteam via injection, but it's not poisonous if eaten. So, the quality of the curare paste could be assessed simply by tasting it without danger, and was described to be a good stomachic (improving stomach function and increasing appetite). But its potency as an arrow poison was varied and needed testing. A frequently used method was testing the distanced travelled by an animal hit by a
curare dart. If a monkey shot by a dart could only get from one tree to the next before dying, this was called a ‘one-tree curare’, the superior grade. ‘Two-tree curare’ was less adequate, and ‘three-tree
curare’ was very weak such that it was used to bring down and sedate animals that the Indians wanted to keep in captivity. Therefore, the potency of the poison is a deciding factor as to whether it kills or not.
So, what is the chemistry happening?
The biosynthesis of tubocurarine (the most important curare) looks rather complicated, but it involves a radical-coupling reaction of the two enantiomers of N-methylcoclaurine, R and S, respectively. To begin with, there is a methyl group present on each nitrogen atom in the two enantiomers of N-methylcoclaurine. Two diradicals are formed by a so-called one-electron oxidation of a free phenol group in each ring. Following this, they then radically couple giving ether bridges. The subsequent addition of a methyl group happens by the addition of S-adenosyl methionine (SAM) forming tubocurarine with the single quaternary N,N-dimethylamino group.
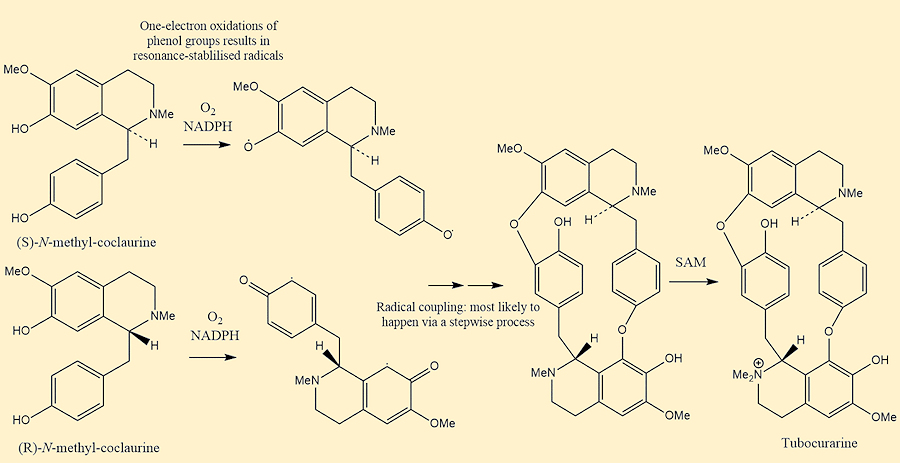
Biosynthesis of Tubocurarine
[Photo: Made in ChemDraw, mechanism taken from P.M Dewick Medicinal Natural Products: A Biosynthetic Approach]
And tubocurarine is the main poison?
Yes, tubocurarine is a prominent representative of the alkaloid class called bis-benzyltetrahydroisoquinoline. It is a cyclic molecule with head-to-tail connected diphenyl ether linkages. It is a salt of a monoquaternary ammonium cation. The quaternary ammonium cations have the structure of [NR4]+ where the R group can be an aryl, alkyl, or organic group, and they are always charged.
As it was used for hunting, it isn’t really used anymore, right?
Well yes and no. By the end of the nineteenth century, the varying properties of
curare were becoming increasingly known to pharmacologists, and therefore it took on a new application and became the first paralytic used in anaesthesia during surgical procedures in 1942. However, it is no longer clinically used, and has been replaced in the medical field by curare-like agents that have fewer side effects.
Wait …. How can it be used as a paralytic if it was used to hunt and kill animals?
Ah, in 1855, Claude Bernard became the chair of Physiology at the College de France and upon experimenting with curare, he saw the results that answer this. The death of the animals struck by arrowheads covered in ‘arrow poison’ was caused by asphyxia as the skeletal muscles relax and then become paralyzed in large enough doses, so the muscles of respiration stop working. However, entry into the bloodstream is needed for the action of the poison to take place. Therefore, if ingested orally it has no ill-effects, assuming there aren’t open sores in the mouth or throat, and its vapours are not poisonous. This is how the Indians were able to use curare as a stomach remedy. |

Claude Bernard
[Photo: Public domain, via Wikimedia Commons] |
Is the death therefore painful?
Death from curare poisoning is caused by respiratory failure. It was reported by Bernard that it happens without convulsions or pain, and that animals did not show signs of nervousness or discomfort. Instead, the main indicator that death induced by curare had occurred, was through muscle paralysis. While pain is not experienced, there is a horror to curare poisoning. Curare does not cross the blood-brain barrier; therefore, the victim is awake and can be aware of what is happening until they lose consciousness. Moreover, the victim can feel the growing paralysis but cannot call out or signal for help. The muscular paralysis caused by curare occurs in a precise order. The first signs of curare poisoning are the eyelids drooping, the victim having a loss of speech, drowsiness and the neck muscles being paralysed. The extremities of the body such as arms and legs are then affected and subsequently the muscles of the diagram. Lastly, death ensues from respiratory failure. However, it is worth noting the heart continues to beat even when respiration ceases, meaning that heart function is not brought to a stop by curare. Therefore, if artificial respiration is performed for a long enough period, revival should occur without negative side effects.
What about it makes it a paralytic?
Experiments performed on frogs by Claude Bernard in 1857 showed the paralysing activity of curare resulted from an interference in the conduction of nerve impulses from the motor nerve to the muscle. In animals treated with curare, the nerve and muscle can still respond to stimuli, however there is a break in the conduction of nerve impulses which occurs at the junction between the two.
In a neuromuscular junction, acetylcholine is a neurotransmitter released from nerve endings into a space called the synaptic cleft which transmits a message from a nerve to the muscle. It then binds to receptors called nicotinic acetylcholine receptors which are present on sodium channels sitting on the muscle's surface. Acetylcholine binds to the receptor and sends a message to the sodium channel resulting in the channel opening causing sodium to go into the muscle. This results in a muscle contraction. Curare acts as a neuromuscular blocking agent. It does this by binding to the acetylcholine receptor, preventing nerve impulses from activating skeletal muscle contractions. The consequence of curare competing with acetylcholine is relaxation and paralysis in lethal doses as the muscle does not contract.
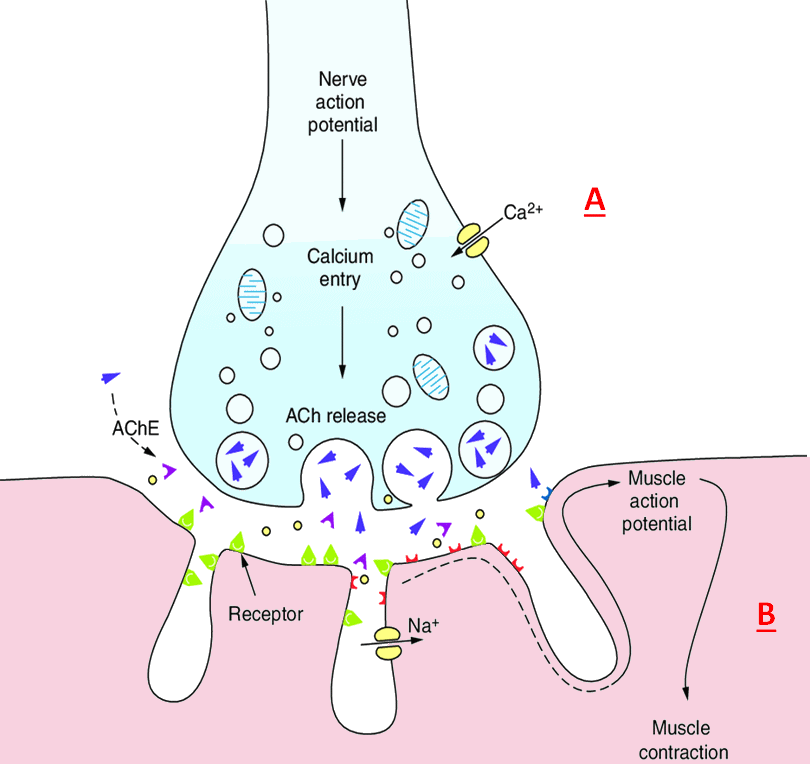
The neuromuscular junction. A signal comes from the motor neuron to the axon terminal (A). There is then an influx of calcium ions.
These calcium ions bind to proteins that sit on the surface of ‘pockets’ which contain the neurotransmitter, acetylcholine (ACh).
Upon binding, the pocket will be drawn to the axon terminal membrane and fuse with it, allowing the acetylcholine to be released into the synaptic gap.
The acetylcholine then travels across the gap and binds to the receptors on the muscle,
allowing sodium to influx into the muscle cell (B). The muscle will then contract.
[Image: Modified from: S. Muppidi, G. I. Wolfe and R. J. Barohn, in Swaiman's Pediatric Neurology: Principles and Practice, Elsevier, Amsterdam, 5th edn, (2012), ch. 91, pp. 1549.]
However, there is a natural antidote for moderate curare poisoning, which are anti-acetylcholinesterase drugs. Examples are physostigmine and neostigmine. In the body, acetylcholine is neutralised by an enzyme called cholinesterase, found in blood and tissues. Physostigmine and other anti-acetylcholinesterase drugs prevent acetylcholine from being neutralised and allows for there to be an excess of acetylcholine at the muscle receptor, which can overcome the blockage at the neuromuscular junction caused by curare being present.
How did curare come to be used in medicine in the west?
When explorers, such as Humboldt, returned home and told people about curare, doctors became interested. Curare was first brought to the US in 1938 by Richard Gill, who lived in Ecuador at the time. Gill had become interested in the medicinal uses of curare after a horse-riding accident ater which he'd developed nerve and muscle damage. Gill's neurologist told him about curare, so he went into the jungle and befriended a tribe who used curare for hunting. The tribes people then showed him how to procure and use it. Gill eventually returned to the US with approximately 25 pounds of curare paste. One of its first uses was during electro-convulsive therapy for treating patients with certain mental illnesses. The electric shocks were so powerful that the patients would have spasms, which could be so violent they often broke bones! Curare, being such a powerful muscle relaxant, lessened the violence of the spasms and prevented bone fractures.

1943 cartoon by Clark Haas showing Richard Gill visiting native peoples to obtain more
curare.
It suggests Gill traded curare for penicillin (MOTM for May 2024) and sulfanilamide (MOTM for July 2011), two antibiotics developed in the west.
[Image: Arthur Guedel collection, MSS 2016-03.]
What is the rationale of it being used in anaesthesia?
As curare is a paralytic, it was used to reduce the need for deep anaesthesia. Heavy doses of toxic anaesthetic agents, which were required previously to create satisfactory operating conditions, often led to post-operative depression, shock, high rates of pulmonary complications and toxaemia (a complication in pregnancy) with signs and symptoms of prolonged nausea and vomiting. Administering curare means a patient only susequently requires a minimal dose of the anaesthetic agent, just enough to sufficiently keep them asleep. Patients are therefore often awake at the end of their operation, being co-operative and fully conscious on return to the wards, and they can breathe freely. These outcomes reduce post-operative complications. Thus, curare’s use in anaesthesia was seen to be effective, as it led to a new conception of 'light anaesthesia', avoiding the unpleasantness experienced through use of deep anaesthesia. This being said, the doses of curare or the anaesthetic agent used in operations must be considered carefully as the combination of curare and deep anaesthesia can be very harmful. A danger in using curare is the diaphragm being paralysed, which results from using larger doses.
|

The unveiling of a plaque on the 10th Anniversary of curare being introduced into clinical anaesthesia.
Photo: A.M. Betcher, Anesthesia & Analgesia, (1977) 56, 305.
|
What doses are given, then?
The dosage of curare is dependent on the individual. Older patients required a lower dose, which also applied to children. While doses were not based on weight in adults, in children, weight was a fundamental factor as the curare dose needed bears a relationship to their muscle mass. The possible dangers of curare led to the administration of a necessary 'induction dose' to observe the individual reactions of different patients. A reported safe induction dose for children was found to be 2 mg per stone body weight, in healthy fit adults 15 mg, and in patients between 65-70 years old, 10 mg was the recommended dose. An initial dose which was one-third of the induction dose, 5 mg in healthy adults, was given intravenously. A pause of three minutes followed, with the remaining induction dose of 10 mg then being administered. The response to the trial dose was patients feeling ‘drowsy,’ ‘heavy’ or weak. A small amount of weakness of the eye muscles was often observed but no definite eye drooping or double vision was to be expected, and especially no difficulty or discomfort in breathing. Reactions which were more serious than these predictions led to curare dosages being modified, or removed completely, depending on how adverse the reaction.
Are there any interesting cases/incidents with curare?
Indeed, there are. Curare was part of an elaborate assassination attempt to kill both Lloyd George, the UK Prime Minister (1916–1922) and Arthur Henderson, the UK Paymaster General (1916-1916). The bizarre plot involved a Mrs Wheeldon, her two daughters, and Mr Mason, her son-in-law who had ownership of a chemist’s shop in Southampton. The entire family were conscientious objectors, who were dissatisfied with the First World War and the political system in general. The plot was foiled because the group of protestors had been infiltrated by a government agent, known as ‘Gordon’, who gained knowledge of the assassination plan. The plot was relayed to the Head of Intelligence, Major Melville Lee, who swiftly deployed a second secret agent, Herbert Booth, to further investigate. Booth was able to win over the conspirators - so much so, that he was given the job of assassin! He was given an airgun loaded with curare pellets and instructed to hide on Walton Heath Golf Course and wait for the Prime Minister. Mason also mailed a parcel to Booth, which contained two tubes of another poison, strychnine (MOTM for October 2009) and two of curare.
 |
 |
The British Prime Minister Lloyd George (left)
and paymaster general Arthur Henderson (right)
[Photo: Bain News Service, Public domain via Wikimedia Commons]
|
Lloyd George on the golf course at Walton Heath.
[Source: Walton Heath Golf Club website] |
Needless to say, the assassination did not take place! Upon examination of the vials by a pathologist, it was found that there was enough of both poisons to kill a few individuals. The four schemers were brought to trial. Mrs Wheelson was given a sentence of ten years, Mr Mason was sentenced to seven years, and Mrs Mason, five. The other daughter of Mrs Wheeldon was acquitted.
Any others?
More recently, in 1976 a formal charge was brought against an Argentinian physician, Mario Enrique Jascalevich following the “Dr. X” killings; several questionable deaths brought about by curare poisoning in 1966 at a Bergen County, New Jersey hospital. Jascalevich was charged with the deaths of five patients having injected them with curare with the intent of murder. It was also recorded that there could have been another nine possible victims ranging in age from 4 to 80. While 18 mostly empty vials of curare were found in the locker of Jascalevich in 1966, he was acquitted by jurors in 1978, after 34 weeks of testimony. Even though he was acquitted, the New Jersey Board of Medical Examiners rescinded Jascalevich's license to practice medicine in the State of New Jersey.
Has curare had any other uses?
Yes, it has been utilised for those suffering with neurological conditions such as multiple sclerosis, tetanus, and Parkinson’s disease. For these conditions, curare temporarily relaxes rigid muscles and manages convulsions.
|

Mario Enrique Jascalevich at his trial
[Photo: AnonymousUnknown author, Public domain, via Wikimedia Commons] |
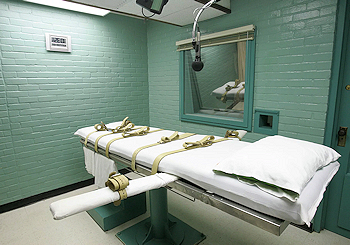 In addition, modified versions of curare have been used in lethal injection for death row inmates in some States of the USA. The lethal injection is comprised of a 3-drug cocktail. The first, an aesthetic causes unconsciousness, the second, a paralyzing agent, causes immobility, and third, potassium chloride, stops the heart. The paralysing drugs are obtained from a curare source or a curare-mimicking agent, and has the intended purpose of paralysing the muscles to stop movement of the unconscious condemned prisoner. It is not needed for the execution to be effective - however, due to the high dosage used, death often comes about from a combination of cardiac arrest and respiratory arrest. This method is frowned upon though, as there is no way to judge if the prisoner is in distress, as the curare-type neuromuscular blocking agent prevents the prisoner showing discomfort or pain (see the quotation, below). Due to this, continuous anaesthesia is needed to prevent extreme suffering.
In addition, modified versions of curare have been used in lethal injection for death row inmates in some States of the USA. The lethal injection is comprised of a 3-drug cocktail. The first, an aesthetic causes unconsciousness, the second, a paralyzing agent, causes immobility, and third, potassium chloride, stops the heart. The paralysing drugs are obtained from a curare source or a curare-mimicking agent, and has the intended purpose of paralysing the muscles to stop movement of the unconscious condemned prisoner. It is not needed for the execution to be effective - however, due to the high dosage used, death often comes about from a combination of cardiac arrest and respiratory arrest. This method is frowned upon though, as there is no way to judge if the prisoner is in distress, as the curare-type neuromuscular blocking agent prevents the prisoner showing discomfort or pain (see the quotation, below). Due to this, continuous anaesthesia is needed to prevent extreme suffering.

Quote about the effects of curare.
[taken from: The Zoophilist: A Catechism of Vivisection. The Whole Controversy Argued in All Its Details
Edward Berdoe, (1883), chapter XVI – curare, 1899, page 173.]
Nowadays, pancuronium bromide is used instead of curare as part of the 3-drug lethal injection process. Although contolling this drug's dosage is more reliable than curare, errors have still been made with other components of the cocktail leading to several civil lawsuits alleging unecessary cruelty.
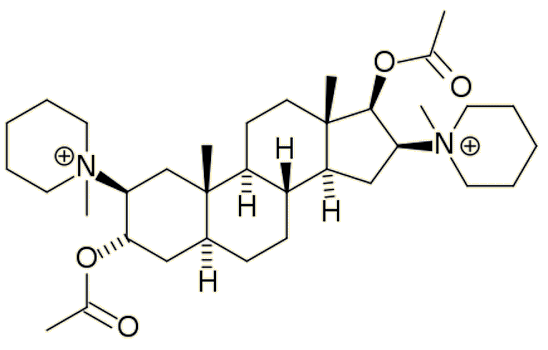 |
| Pancuronium bromide |

Bibliography
Wikipedia: Curare, Tubocurarine_chloride; Toxiferene; Arrow poison; Quaternary_ammonium_cation; Dr._X_killings
- A.E. Rigby-Jones, S.A. Burr, Encyclopedia of Toxicology (Fourth Edition), (2024) 3, 353-357.
- S.A. Burr, Y.L. Leung, Encyclopedia of Toxicology (Third Edition), (2014) 1088-1089.
- L.E. Craig, The Alkaloids: Chemistry and Physiology, (1955) 5, 265-293.
- J. Carl, M. Schwarzer, D. Klingelhoefer, D. Ohlendorf, D. A. Groneberg, PLoS One, (2014) 9(11).
- M. Heinrich, Comprehensive Natural Products II, (2010) 3, 351-381.
- MR Lee, J. R. Coll. Physicians Edinb., (2005) 35, 83-92.
- Wayback Machine – Internet Archive, Curare, (accessed January 2024).
- P.M Dewick, in Medicinal Natural Products: A Biosynthetic Approach, John Wiley & Sons Ltd, Chichester, 3rd edn, 2009, ch.6, 311-420.
- C. Weber, T. Opatz, The Alkaloids: Chemistry and Biology, (2019) 81, 1-114.
- R. West, Proc. Roy. Soc. Med., (1935), 28(5), 565-578.
- S.K. Bardal, J.E. Waechter, D.S. Martin, Appl. Pharmacol., (2011), 325-365.
- M. Heinrich, Compr. Nat. Prod. II, (2010), 3, 351-381.
- T.C. Gray, Ann. R. Coll. Surg. Engl., (1947) 1(4) 191–203.
- Khan Academy, Neuromuscular junction, (accessed March 2024)
- D. E. Hale, Cleveland Clin. Q., 1946, 13(30), 177-182.
- H. G Kinnell, BMJ, 2000, 321(7276), 1594–1597.
- J. Berger, The New York Times, (1985) Dr. Mario E. Jascalevich Dies; Jersey Surgeon in 'Dr. X' Case, (accessed March 2024).
- D. Kroll, Forbes, (2014) The Drugs Used In Execution By Lethal Injection, (accessed February 2024).
- O. Dyer, BMJ, (2014), 348.
- T.A. Zimmers, J. Sheldon, D.A. Lubarsky, F. López-Muñoz, L. Waterman, R. Weisman , L.G. Koniaris, PLoS Med, (2007) 4(4), e156.
- A. Labidi, Chemicals in Lethal Injection, 2020.


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.25764714]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.25764714]

![]()
![]()
![]()
![]()
















 In addition, modified versions of curare have been used in lethal injection for death row inmates in some States of the USA. The lethal injection is comprised of a 3-drug cocktail. The first, an aesthetic causes unconsciousness, the second, a paralyzing agent, causes immobility, and third, potassium chloride, stops the heart. The paralysing drugs are obtained from a curare source or a curare-mimicking agent, and has the intended purpose of paralysing the muscles to stop movement of the unconscious condemned prisoner. It is not needed for the execution to be effective - however, due to the high dosage used, death often comes about from a combination of cardiac arrest and respiratory arrest. This method is frowned upon though, as there is no way to judge if the prisoner is in distress, as the curare-type neuromuscular blocking agent prevents the prisoner showing discomfort or pain (see the quotation, below). Due to this, continuous anaesthesia is needed to prevent extreme suffering.
In addition, modified versions of curare have been used in lethal injection for death row inmates in some States of the USA. The lethal injection is comprised of a 3-drug cocktail. The first, an aesthetic causes unconsciousness, the second, a paralyzing agent, causes immobility, and third, potassium chloride, stops the heart. The paralysing drugs are obtained from a curare source or a curare-mimicking agent, and has the intended purpose of paralysing the muscles to stop movement of the unconscious condemned prisoner. It is not needed for the execution to be effective - however, due to the high dosage used, death often comes about from a combination of cardiac arrest and respiratory arrest. This method is frowned upon though, as there is no way to judge if the prisoner is in distress, as the curare-type neuromuscular blocking agent prevents the prisoner showing discomfort or pain (see the quotation, below). Due to this, continuous anaesthesia is needed to prevent extreme suffering.
