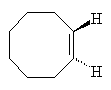
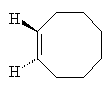
 Cyclooctene
Cyclooctene

 Cyclooctene
Cyclooctene
On one hand, it would seem that nature could simply choose either chiral form of any molecule to play with. Many molecules can exist in two so-called handed or chiral forms - each, known as an enantiomer, is a non-superimposable mirror image of its counterpart - like a pair of hands. To the chemist this handedness in nature is more than academic one handed form of a molecule will react with another chiral molecule differently from the opposite enantiomer. To the pharmacologist, one enantiomer of a drug may interact differently with the receptor or enzyme in the body (which are themselves chiral) and so have different effects. One enantiomer of the painkiller ibuprofen is three times stronger than its mirror image while one form of some drugs are very effective and the other have dangerous side effects.
In the 1980s, chemist William Bonner of Stanford University and Edward Rubenstein of Stanford's Medical School in the USA came up with a peculiar theory to explain why nature opted for one chiral form and not the other. Until now, however, there was little more than circumstantial evidence to support their idea.
The researchers first assumed that life could not have arisen if nature had tried to work with both chiral forms. There is experimental evidence that shows that proteins cannot be built nor cells multiply if both chiral forms of DNA are present, for instance. The team then looked to the stars for a possible trigger that could have fired the starting gun by forcing nature to choose between left and right since chirality could not they say have arisen spontaneously in a chemical reaction on the early Earth.
Neutron stars throw out electromagnetic radiation which is circularly polarised anticlockwise from the star's northern hemisphere and clockwise from the south - the radiation is chiral. Bonner and Rubenstein reckon this chiral radiation would influence the chemistry of local interstellar dust resulting in an excess of one set of enantiomers of molecules passing by the northern hemisphere and the opposite from the south. Dust from one hemisphere of a neighbouring neutron star should it have reached the young earth would have been enough chirality to have seeded the pre-biotic chemistry that gave rise to life.
Yoshihisa Inoue and his colleagues at the ERATO Photochirogenesis Project at Osaka University working with teams from Himeji Institute of Technology and Umezono of Tsukuba have now developed a synchrotron radiation source that produces polarised ultra-violet light of either left or right-handed form.
They have used this chiral radiation to mimic the effects of one hemisphere of a neutron star on a chemical reaction to see whether they could force it to go down only one of the two possible paths to left or right-handed product.
They picked a simple reaction which can be driven by light. In the reaction the double bond between two of the carbon atoms in the cyclic hydrocarbon cyclooctaene is made to flip around so that the product has a twist. Depending on which way the bond flips produces either a left-handed or a right-handed product. In normal UV light both products form in equal amounts.

| 
| 
|
When Inoue and his colleagues used their chiral UV they produced an excess of one form of a mere 0.12%. This they claim, however, is sufficient bias to provide proof of an extraterrestrial origin of the single-handedness of chemistry in nature.
The possibility of influencing industrially important reactions such as synthesis of single enantiomers of drugs and catalysts might now be possible as spin off of the rotating UV light. Although it remains to be seen whether the percentages could be raised or whether the reactions would take millions of years.
This page originally appeared as an article in David Bradley's Elemental Discoveries.
The usual disclaimers apply, which means if you play with synchrotron radiation and hydrocarbons you do so at your own risk.
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5437429]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5437429]