![]()
   |
Dettol |    |
![]()
![]()
Also available: HTML-only, VRML and Jmol versions.
![]()
 Dettol is one of those chemicals which we instantly recognise by its distinctive smell. It is an aromatic compound derived from phenol, which contains a significant chlorine atom, helping us in our continuous fight against unwanted bacteria. Aromatic compounds are a good way to get people interested in Chemistry, particularly as those with three identical groups present, e.g. the fungicide trichlorophenol (TCP) (not to be confused with the household germicide TCP, trichlorophenylmethyliodosalicyl) which, like dettol, has a characterisitic phenolic odour, and the explosive TNT (trinitrotoluene) provide the general public with yet more TLA's (three letter acronyms).
Dettol is one of those chemicals which we instantly recognise by its distinctive smell. It is an aromatic compound derived from phenol, which contains a significant chlorine atom, helping us in our continuous fight against unwanted bacteria. Aromatic compounds are a good way to get people interested in Chemistry, particularly as those with three identical groups present, e.g. the fungicide trichlorophenol (TCP) (not to be confused with the household germicide TCP, trichlorophenylmethyliodosalicyl) which, like dettol, has a characterisitic phenolic odour, and the explosive TNT (trinitrotoluene) provide the general public with yet more TLA's (three letter acronyms).
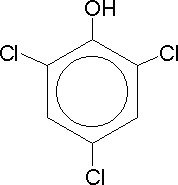 Trichlorophenol |
|
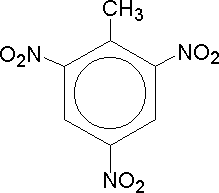 TNT |
How many A-level students could correctly give the IUPAC name for dettol? Its structure follows, and it should be helpful to remember both the alphabetical rule and that an aromatic ring with an OH group is called a phenol. Now you should arrive successfully at the name 4-chloro-3,5-dimethylphenol which is the molecule dettol.
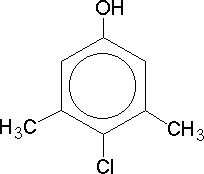 Dettol |
Only 4.8% of dettol consists of this molecule (C8H9ClO) with CAS number 88-04-0, the rest being made up of Pine Oil, isopropyl alcohol, castor oil soap, caramel and water. The molecule forms white/cream coloured crystals with m.p. 114-116°C and has an oral LD50 of 3.83 kg/kg for a rat's body - a reassuring fact, even if this way of quoting toxicity levels is currently out of fashion.
 Dettol cream |
 Dettol soap |
 Dettol plasters |
Dettol is made by Reckitt & Colman and is commercially available as an inexpensive liquid antiseptic which is safe and gentle enough to use on the skin and yet powerful enough to also use as a disinfectant. This is because of its broad spectrum of antimicrobial action. It is effective against gram positive/negative bacteria, fungi, yeast, mildew and even the frightening "super-bug" MRSA. It is able to kill 98% of microbes in just 15 seconds as shown in agar patch studies. Dettol is found in a number of products under a number of different names, for example as 0.5% chloroxylenol in the powder Zeasorb used in the treatment of athletes' foot, and as the quaintly named parachlorometaxylenol in Vionex the antimicrobrial toutelles. To keep teenagers on board, it is even used to treat acne, which is caused by bacterial infections and not the over indulgence of the irresistible chemicals found in chocolate.
Before you start swilling molecules of dettol around the house like many other chemicals, nbe aware that over-use may lead to contact dermatitis or even bacterial resistance. There is an increased awareness of the problems associated with drug resistant strains of bacteria particularly in hospitals. Chemicals such as triclosan target lipid synthesis in their fight against microbes. Frequent washing with cheaper "plain" soap and water sharply reduce the risks of infection, and good old fashioned but highly effective dettol is a very good way to disrupt a bacterial cells membrane potential, drastically affecting its ability to produce ATP and thus leading to its rapid death.
Dettol has some considerable advantages; from low toxicity, low metal corrosivity (important when sterilizing medical instruments), and is active over pH's 4-9. Despite being only slightly soluble in water it has a good solubility in alcohols (ethanol/propan-2-ol 50-87 g/100 ml) and pine oils. It is cheap at £6.59 / 500 ml. It may not be the most glamorous Molecule of the Month, but it is another example of how a relatively simple molecule has greatly benefited mankind. So the next time someone says how bad Chemistry and the Chemical industry is, why not reply to them with tales of the bacterial power of dettol whilst casually using one of its synonyms; chloroxylenol, 4-chlorometaxylenol, 4-chloro-3,5-xylenol, and of course, confusing to the under 30's parachlorometaxylenol (PCMX).
![]()