Dioxin
(2,3,7,8-TCDD)
by
Fabio Pichierri
Molecule of the Month, September 2005.
Introduction
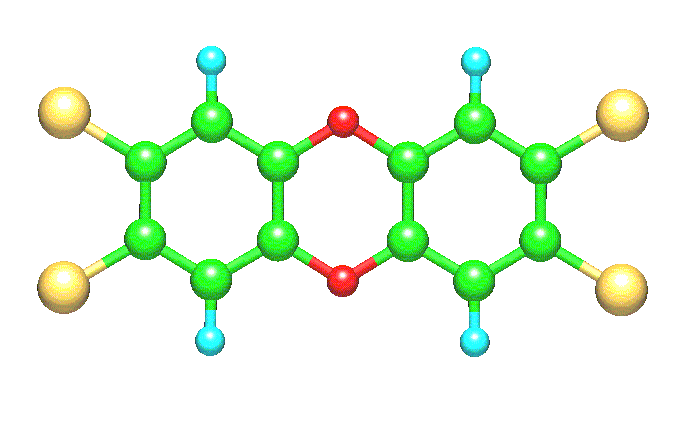
Dioxin, the short name
for 2,3,7,8-tetrachlorodibenzo-para-dioxin (2,3,7,8-TCDD), is considered to be one of the most
dangerous compounds that pollute our environment. Its chemical structure is
made of two aromatic rings joined through a pair of oxygen atoms, as shown in
the above drawing. Four chlorine atoms, two on each aromatic ring, are attached
at positions 2, 3, 7, and 8. The whole molecule is flat-like and possesses D2h
symmetry. If we consider that up to eight chlorine atoms can be attached to the
dibenzodioxin (DD) skeleton, then 75
chlorine-substituted DD isomers can be conceived. Dioxin is inextricably linked
to environmental pollution from waste incineration and to its incidental
formation in chemical plants that are devoted to the production of pesticides [Tuppurainen 2003].
A man-made disaster
Here we give a short
account of the Seveso incident which happened almost
thirty years ago in northern
The morning of July 10th (Saturday) 1976 is an ordinary morning just
like any other. At
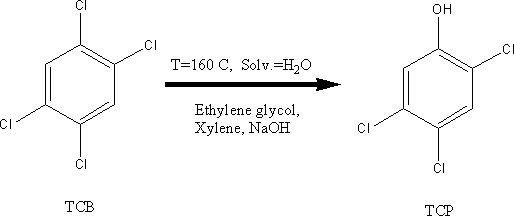
The exothermic reaction
increased excessively the pressure of the vessel containing the reactants and,
as a result of the malfunctioning of a safety valve, several compounds among
which about 30 Kg (!) of TCDD escaped directly into the atmosphere. In the days
following the incident the authorities had to estimate the real extent of the
disaster so as to quickly undertake the necessary procedures of evacuation,
medical assistance, along with securing the contaminated area. Emergency
procedures are always based on technical and scientific data, such as the
measurement of the amount of TCDD in the blood, fruits, vegetables, and the
soil. This, however, was the first large-scale environmental disaster concerned
with TCDD contamination and little or no information was available at that
time. As Prof. Mocarelli points out, <<Science admits its ignorance within
its realm. To admit it publicly is more difficult>>.
Several committees were
organized and a great deal of scientific and technical activity was undertaken.
The immediate effects of TCDD on humans were (a) lesions of the skin as a
result of contact with the various compounds present in the toxic cloud, and
(b) chloracne,
an acne-like skin condition that results from the exposure to chlorinated
hydrocarbons. Chloracne manifests itself with the
formation of small bumps, termed comedomes, and cysts
on the cheeks and behind the ears. Statistical studies performed over several
years on pregnant women did not show a significant increase in malformations
although the sex ratio at birth appears being skewed toward females.
Furthermore, an increase in the risk of tumors of the lymphoid system has
also been observed. A comprehensive toxicological study of the Seveso incident has been published in 2003 [Pesatori et al. 2003].
Besides the medical
aspects related to the incident, an important problem that had to be solved at
that time was cleaning the contaminated areas near Seveso
and Meda. Incineration
was initially suggested and supported by both scientists and politicians.
Eventually, under the pressure of the public opinion, the authorities opted for
soil scarification and its disposal into
specially-constructed basins. An
Toxicology
It has been established
that TCDD binds to the aryl hydrocarbon receptor (AHR)
in human tissues [Mandal 2005]. From here, the
AHR-TCDD complex enters the cell nucleus to interact with a specific DNA
sequence. The complex is believed to act as a transcription factor of the
alpha-beta-alpha family that initiates a signaling cascade which provokes the
observed tissue changes (e.g. chloracne). The
characterization of the 3D structure of AHR (or part of it) will greatly help
in shading further light on the molecular mechanisms behind the toxicological
effects of TCDD (and other chlorinated hydrocarbons) as well as in finding
possible remedies (drugs, therapies, etc) for the treatment of dioxin
poisoning.
Electronic structure
The electronic structure
of molecules arises from the physics of both electrons and nuclei. Within the
framework of quantum mechanics, the branch of physics devoted to the study of
microscopic particles, the time-independent non-relativistic Schrödinger equation (Hy=Ey)
coupled to the Born-Oppenheimer approximation
(stating that the motion of electron can be decoupled from that of nuclei as a
result of their different masses) represent a good starting point for modeling the
electronic structure of polyatomic molecules. The figure below shows the plots
of four molecular orbitals
(MOs) of TCDD whose (eigen)energies
are the numerical solutions of the Schrödinger equation. The MOs spanning the
HOMO (highest occupied MO) and LUMO (lowest unoccupied MO) levels are those of
interest to research chemists for they are related to important properties of
the molecule such as its chemical reactivity. On the top-left side, the HOMO-1
level which shows two pair of "lips" arising from the combination of carbon p-type
atomic orbitals lying perpendicular to the molecular
plane. The HOMO level (top-right) shows the contribution of four pairs of
p-type atomic orbitals of carbon atoms. Both HOMO and
HOMO-1 possess anti-bonding character with respect to the central C—O
bonds.
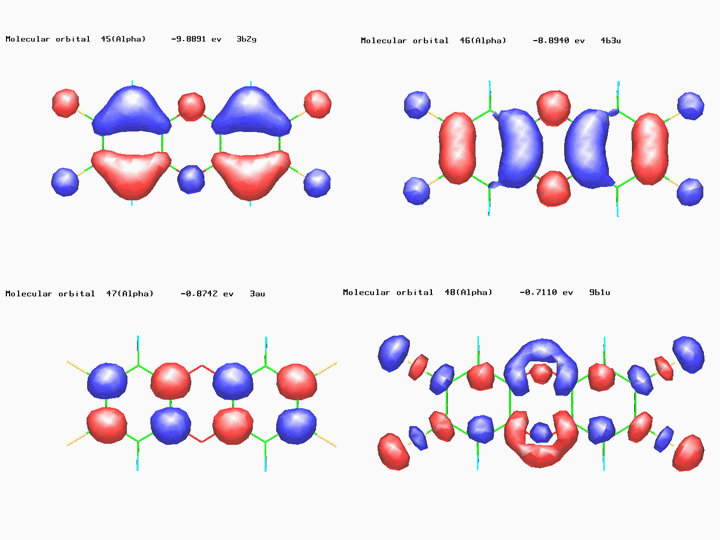
On the bottom-left side,
the LUMO level which arises from the p-type orbitals
of eight carbon atoms and possesses anti-bonding character with respect four C—C
bonds. The LUMO+1 level (bottom-right) displays the contributions from the
p-type orbitals of chlorine atoms and has anti-bonding
character with respect to the four C—Cl
bonds. Populating it with electrons (upon either chemical or electrochemical
reduction of TCDD) might help in achieving (partial or total) de-chlorination
of TCDD [the calculations were carried out by me using Stewart's PM3 semiempirical MO method as implemented in the WinMOPAC software package].
Several physico-chemical properties have been predicted from the
results of electronic structure calculations (computational quantum chemistry).
For example, the adiabatic electron affinity (EA)
of TCDD corresponds to 0.259 eV as computed at the
B3LYP/aug-cc-pvDZ level of theory [Arulmozhiraja et
al. 2000]. Furthermore, theoretical calculations performed on the simple DD molecule
(with hydrogen atoms in place of chlorine atoms) predict that the replacement
of both oxygen atoms with sulfur and selenium produces puckered molecules
characterized by high inversion barriers [Kim et al. 2003]. The results have
been interpreted as being a direct consequence of the electronic structure of
these molecules.
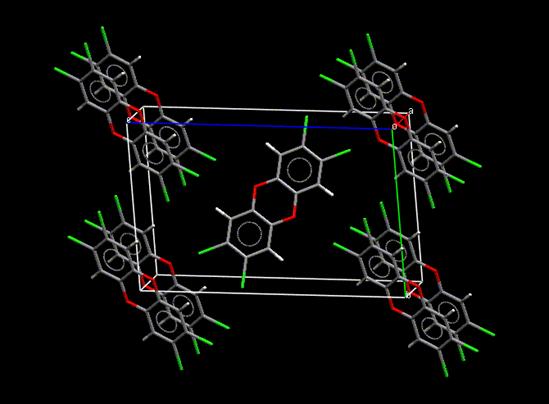
Crystallography
The molecular structure
of TCDD has been experimentally determined by single-crystal x-ray diffraction crystallography [Boer 1973]. As shown in the
figure below, the packing of TCDD in the crystal shows the herringbone motif observed in the molecular crystals
of polyaromatic hydrocarbons (PAHs).
The molecules in the crystal are characterized by Cl•••O contacts at 3.087 and
3.131 Å. These and other crystallographic data are available from the Cambridge
Structure Database (CSD) which is being distributed by the Cambridge Crystallographic Data Centre (CCDC).
References
K. Tuppurainen et al., Perspectives
on the Formation of Polychlorinated Dibenzo-p-dioxins
and Dibenzofurans during Municipal Solid Waste (MSW)
Incineration and Other Combustion Processes, Acc. Chem. Res. 36 (2003) 652-658
P. Mocarelli,
Seveso: a teaching story, Chemosphere 43 (2001) 391-402
A.C. Pesatori
et al., Short- and long-term morbidity
and mortality in the population exposed to dioxin after the Seveso accident,
Ind. Health. 41
(2003) 127-38
P.K. Mandal,
Dioxins: a review of its environmental effects and its aryl hydrocarbon
receptor biology, J. Comp. Physiol. B 175 (2005)
221-230
S. Arulmozhiraja et al., Electron affinities for the most
toxic 2,3,7,8-tetrachlorodibenzo-p-dioxin: a density functional theory
study, J. Phys. Chem. A 104 (2000)
7068-7072
S. Kim et al., A theoretical
investigation into the conformational changes of dibenzo-p-dioxin, thianthrene, and selenanthrene, J. Mol. Struct. (Theochem) 655 (2003) 451-458
F.P. Boer et al., Adv. Chem. Ser. 120 (1973) 14
Books
Dioxins
and Health, by Arnold
Schecter and Thomas A.
Gasiewicz, Wiley-Interscience;
2nd edition (
Mechanistic Aspects of the Thermal
Formation of Halogenated Organic Compounds Including Polychlorinated Dibenzo-p-Dioxins (Current Topics in Environmental and
Toxicological Chemistry), by G.G. Choudry and O. Hutzinger, Gordon & Breach
Science Pub (August 1, 1983)
The Dioxin War: Truth and Lies about a
Perfect Poison, by Robert Allen, Pluto Press (
La Fabbrica dei Profumi (The Fabric of
Perfumes), by Daniele Biacchessi, Baldini & Castoldi, 1995.
[This book recounts the story of Seveso's incident
and is available in Italian only]
Useful links
The official site of the
town of
DIMESAB (Dipartimento di medicina
ambientale sperimentale e biotecnologie sanitarie)
at the
Society of Toxicology: http://www.toxicology.org/
DioxinFacts.org: http://www.dioxinfacts.org/index.html
US Environmental
Protection Agency (EPA): http://www.epa.gov/
An informative home page
about dioxin: http://www.ejnet.org/dioxin/
A resource guide on skin cancer awareness: https://thedermreview.com/skin-cancer-awareness-guide/
Dioxin 2004
symposium: http://www.dioxin2004.org/frameset.htm
This
web page is dedicated to the memory of my dear uncle who sparked my interest
for Science and the mysteries of Nature as a mean to minimize my ignorance
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5436814]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5436814]