![]()
Estradiol
The main female hormone
![]()
![]()
Molecule of the Month July 2019
Also available: JSMol version.
![]()
EstradiolThe main female hormone
Molecule of the Month July 2019
|
 |
No, estrogen is actually the name given to a group of compounds, including estradiol, that are important in the female reproductive cycle. The name comes from the Greek oistros meaning ‘producer of sexual desire’. Like the male equivalent, testosterone (see MOTM page for August 2019), estrogens are produced in all vertebrates and insects, which suggest that they appeared very early on in the evolution of life on Earth.
Yes, in both the gender and reproductive meaning of the word. The sex hormones determine whether an animal is male or female, while the relative amounts of the different sex hormones determine the sexual characteristics of that animal, such as how masculine or feminine they appear and behave.
That’s the most important one, along with estrone and estriol which are less potent. They are all based on the standard 4-ring steroid structure, with just minor differences in side-groups. And this includes testosterone, too. It’s quite amazing that all the differences between male and female come down to something as trivial as the side-group of a molecule!
Although it can be produced by many cells in the body, including fats cells, in the brain and in artery walls (in males as well as females), most estradiol is synthesised in the ovaries from compounds derived from cholesterol (see MOTM for March 2004).
It helps a woman prepare for pregnancy, by supporting the reproductive organs, keeping the eggs healthy in the ovaries, and instigating the monthly ovulation and menstrual cycle. It is also responsible for development of the female secondary sex characteristics, which begin at puberty and decline after the menopause. For example, estradiol initiates development of breasts, and alters the fat distribution in a woman’s body to make her more ‘curvy’. It also helps strengthen bones and joints.
Is that why post-menopausal women have brittle bones?Partly, yes. After the menopause, women’s estrogen levels drop dramatically, and this results in their bones becoming weak and porous, a condition called osteoporosis. To combat this, post-menopausal women, or those who have had a hysterectomy, are often prescribed hormone replacement therapy (HRT), which usually involves ingesting a mixture of estrogen, progesterone, and progestin to compensate for the amount they’ve lost. One common estrogen that’s used in HRT has the trade-name Premarin, and is isolated from the urine of pregnant mares. The name comes from pregnant mares’ urine, and is actually mostly composed of estrone sulfate. It may sound an odd thing to take, but this is converted directly into normal estradiol in the woman’s body. |
 Look what HRT did for me! |
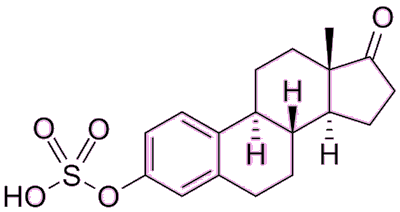 |
| Estrone sulfate |
Exposure to any of the components of estrogen by males can cause anorexia, vomiting and feminisation, such as breast growth, and also erectile dysfunction. In the developed world, sperm counts have been falling by about 1-2% every year for the past several decades. The reason for this is not entirely clear, but one possibility is that men are inadvertently ingesting small quantities of estrogens or estrogenic-like compounds, and these are damaging male fertility. Various chemicals have been blamed for possibly mimicking the effects of estrogen, including bisphenol A, polychlorinated biphenyls and phthalates.
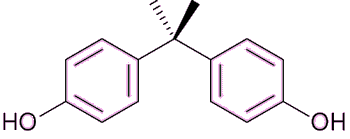 |
Bisphenol A (BPA) (see MOTM for August 2013) is used to make polycarbonate plastic and the epoxy resins that are used as a lining in most food and beverage cans. |
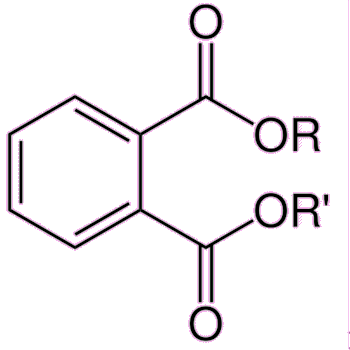 | Phthalates are plasticizers providing durability and flexibility to plastics such as PVC. High-molecular-weight phthalates are used in wall coverings, flooring, and intravenous bags and tubing. Low-molecular-weight phthalates are found in cosmetics such as lotions and perfumes, and also varnishes and lacquers. |
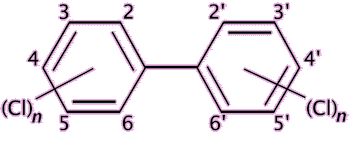 |
Polychlorinated biphenyls (PCB) are chemically stable have low flammability and are electrical insulating, and their main use is as insulating fluids and coolants. |
But another route by which estrogens have made their way into the water supply is as a result of the disposal of unwanted or out-of-date steroid pills and contraceptive pills down the sink or toilet.
Really? How so?The female contraceptive pill contains the hormone progesterone (often called the pregnancy hormone) combined with an estrogen, usually a derivative of estradiol called ethynyl estradiol (EE). High levels of progesterone such as those in the pill are usually only found when a woman is pregnant, so the pill works by tricking the body into thinking it’s already pregnant. The female body’s response to being pregnant is complex. But, amongst other effects, further ovulation is prevented (because no more eggs are needed if one’s already been fertilised), the mucus in the cervix thickens making it difficult for additional sperm to enter, and the lining of the womb becomes thinner so it is less likely to accept (another) fertilised egg. Other contraceptive pills, such as the progesterone-only pill (or mini-pill) contain only synthetic progestogens (compounds chemically related to progesterone but not progesterone itself, despite the name) and no estrogen. Nevertheless, all of these pills contain compounds that mimic estrogen and which can have hormone-disruptive effects on both wildlife and humans. |
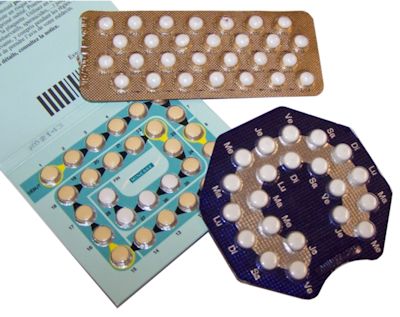 Different types of contraceptive pill. Photo: Ceridwen [CC BY-SA 2.0 fr] via Wikimedia Commons. |
Should we be worried?Well, yes. The male-to-female ratio in many animals and fish species have reportedly been altered by exposure to these chemicals. In some of these cases the males have been ‘feminised’, and have partial female characteristics. And in humans, they are thought to be responsible for premature puberty in regions where high levels have been found in the local environment. Several countries have already started to ban these estrogenic compounds. For example Canada, the US and the European Union have recently (2012) banned the use of BPA in baby bottles. PCBs were banned in 1979 but do not degrade very effectively, so continue to persist in the environment. But there are many more natural and man-made estrogenic compounds all around us, the effects of which we know little or nothing about. Maybe everyone should all start to get used to wearing skirts and dresses..! |
 The ultimate result of estrogen compounds getting into the environment? |
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.7637696]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.7637696]