![]()
ETHYLENE GLYCOL
(ETHANE-1,2-DIOL)
and antifreeze poisoning
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month June 2018
Also available: JSMol version.
![]()

 |
ETHYLENE GLYCOL
|
 |
Yes, that is one of the major uses of ethylene glycol (the other is making polymers).
It lowers the freezing point of water (and raises the boiling point) and is also miscible with water in all proportions. And that is down to the properties of the molecule, particularly its ability to form hydrogen bonds.
Why is that?First, ethylene glycol contains polar O-H groups; they are polar because oxygen is a lot more electronegative than hydrogen, so it tends to polarise the electron pair in the O-H bond towards it. That in turn causes the oxygen to carry a partial negative charge (δ-) and hydrogen a partial positive charge (δ+). Because opposite charges attract each other, this means that ethylene glycol molecules are attracted to each other, making it harder to pull them apart (think of ‘Molecular Velcro’) and this, in turn, makes its boiling point higher than that of hydrocarbons of similar mass. |
|
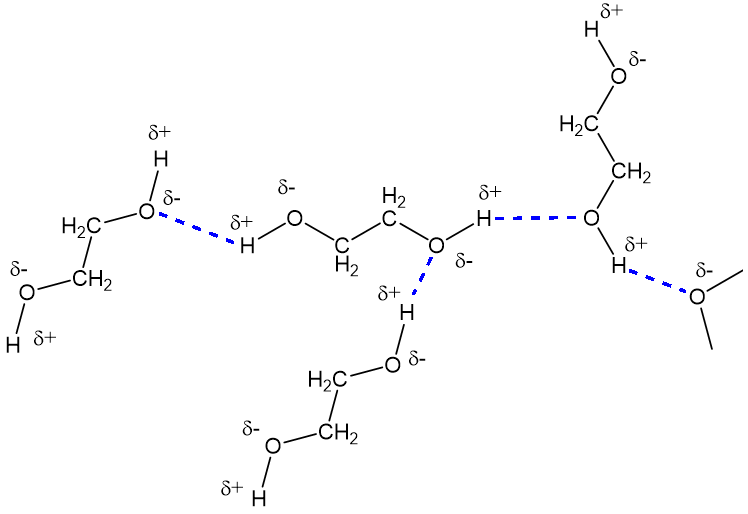
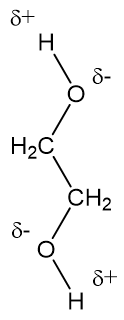
The hydrogen bonding in ethylene glycol.
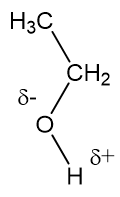
Because ethylene glycol has two –OH groups, both of which can form hydrogen bonds, compared to the one in the other two-carbon molecule, ethanol (‘alcohol’), this is why the boiling point of ethylene glycol is quite a lot higher than that of ethanol.
 |
 |
| Ethylene glycol | Ethanol |
It’s also the increased number of hydrogen bonds in ethylene glycol that makes it more viscous (less runny) than ethanol (or water).
Water also contains O-H groups, of course, which ‘hydrogen bond’ to each other, causing water to have a very high boiling point for a molecule of its size. Thus, ethylene glycol and water molecules can form hydrogen bonds to each other, just as the individual molecules can, which means that they mix freely, in all proportions, as shown in the diagram below.
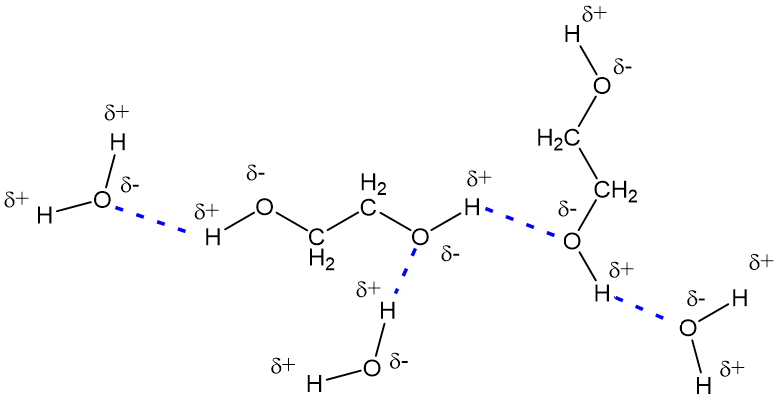
 How does this help with antifreeze?
How does this help with antifreeze?Well, ethylene glycol interferes with the hydrogen bonding network in pure water. Water freezes at 0°C and pure ethylene glycol at -12°C, but a mixture of the two freezes at a much lower temperature – the lowest f.p. reached is -55°C in mixtures containing 70% ethylene glycol. This means that if such a mixture is used in the cooling system of a car, it will not solidify even in very cold winter temperatures. This property is also utilised in de-icing fluid on windscreens or outer surfaces of aircraft.
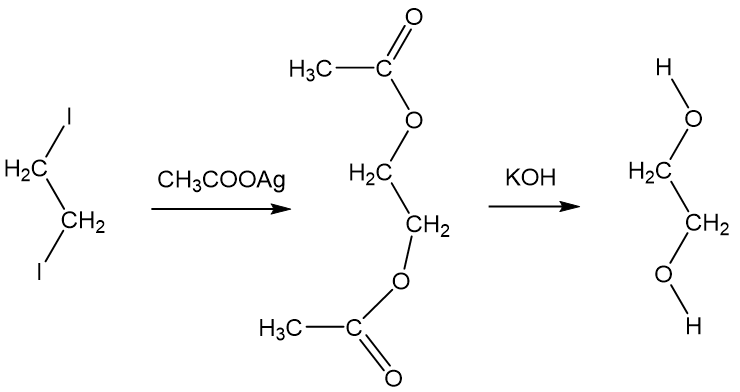
Usually routes start from ethene (MOTM December 2006). It was first reported in 1856 by Adolphe Wurtz (image, right), who was an Alsatian French chemist.
Very far from it. No, he came from Alsace; that’s why there’s a statue of him in Strasbourg. He was a great proponent of atomic theory. Anyway, back to glycol. He reacted 1,2-diiodoethane (indirectly derived from ethene) in an esterification reaction with silver ethanoate (acetate) in what is also a nucleophilic substitution reaction. The resulting ester was then hydrolysed with potassium hydroxide.
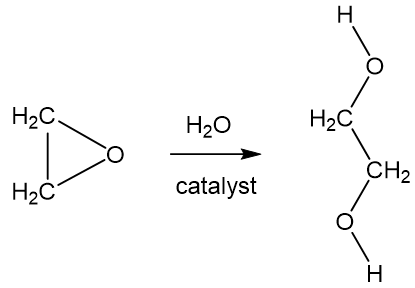
Nowadays commercial manufacture tends to use processes involving the hydration of ethylene oxide, a readily available chemical made from ethene, in turn obtained by cracking alkanes. This process goes back to a discovery that Wurtz reported in 1859.
Yes, it is used as one of the two starting materials used in the manufacture of polyethylene terephthalate (PET, a.k.a. Dacron and Terylene, recycling symbol “1”). The diacid terephthalic acid (benzene-1,4-dicarboxylic acid) reacts with the diol ethylene glycol forming the polyester.
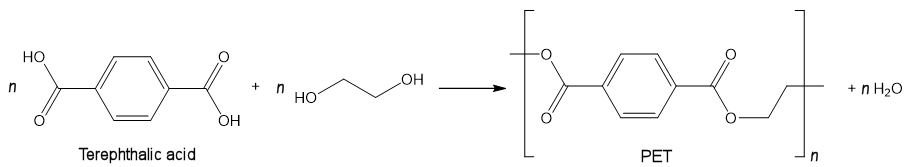
 This has many uses, one being to make plastic soft-drink bottles (which may be used to incorrectly store antifreeze). Others include insulation and wrapping; ropes; carpets and clothing.
This has many uses, one being to make plastic soft-drink bottles (which may be used to incorrectly store antifreeze). Others include insulation and wrapping; ropes; carpets and clothing.
Over 20 million metric tonnes of ethylene glycol are manufactured each year. It can be converted into several other useful chemicals, like glyoxal, glycolic acid and methyl glycolate, and other uses continue to develop, including the synthesis of nanomaterials.
For one thing, it is toxic.
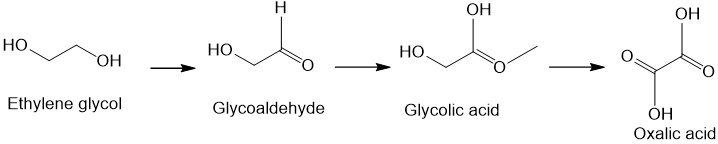
In the liver, the enzyme alcohol dehydrogenase converts ethylene glycol first into glycoaldehyde and then into other toxic molecules. Ultimately oxalic acid removes calcium from the body as insoluble calcium oxalate, which is deposited in the kidneys and damages them, leading to renal failure. Methanoic (formic) acid is also generated, which can cause blindness.
Because antifreeze is such an everyday chemical, some people get careless with it around the home. They store it in containers meant for other things, like containers meant for drinks – and not correctly labelled. People leave it around on garage shelves where children can find it. Spills are not always cleaned up. This means that pets and small children are especially at risk. A spoonful or so can be fatal to a cat or small dog. The lethal dose for an adult human is around 120 grams. Antifreeze usually is brightly coloured and the ethylene glycol gives it a sweet taste, making it attractive to young people. In some places a bitter tasting substance like denatonium benzoate is added to it to put people off, but some research has suggested that this is not successful.
 So it is possible for someone to be poisoned with antifreeze?
So it is possible for someone to be poisoned with antifreeze?Never mind poisoning, there’s quite a list of people murdered with antifreeze. In 2007, an American woman named Lynn Turner (photo, left) was convicted of the 2001 murder of her boyfriend, police officer Randy Thompson, by putting antifreeze in his Jell-O. She had murdered her then husband, firefighter Maurice Glenn Turner, the same way in 1995. Ms Turner received a life sentence, and died in prison in 2011 from an overdose of her prescribed medication. In 2007, a New Jersey woman named Maryann Neabor received a 20-year sentence for killing her brother-in-law by adding ethylene glycol to his cocktail of pineapple juice and maraschino cherries, and making an “antifreeze smoothie”. A Stoke-on-Trent (UK) woman named Kate Knight was found guilty in January 2008 of trying to kill her husband, Lee, by putting antifreeze in his curry and red wine and given a 30-year sentence. Though he survived, Lee had serious sight and hearing loss, and badly damaged kidneys. He subsequently found love with the dialysis nurse Jackie Evans who treated him, and remarried. That same year James Keown of Waltham, Massachusetts, was found guilty of first degree murder, convicted of lacing his wife Julie’s Gatorade with antifreeze.
 Then in 2013, a breast-cancer oncologist, Dr Ana Maria Gonzalez–Angulo (photo, right), who worked at a cancer centre in Houston, Texas, laced the coffee of her lover Dr George Blumenschein (a lung, head and neck cancer specialist) with antifreeze. He commented on the sweet taste but was told that it was Splenda. He survived, with kidney damage. She received a 10-year term for aggravated assault. In 2016, Diane Staudte and her daughter Rachel, from Springfield, Missouri, were both given life sentences after admitting to killing first her husband, Mark, and then her son, Shaun, with antifreeze. Next her elder daughter Sarah became seriously ill, for the same reason, but survived.
Then in 2013, a breast-cancer oncologist, Dr Ana Maria Gonzalez–Angulo (photo, right), who worked at a cancer centre in Houston, Texas, laced the coffee of her lover Dr George Blumenschein (a lung, head and neck cancer specialist) with antifreeze. He commented on the sweet taste but was told that it was Splenda. He survived, with kidney damage. She received a 10-year term for aggravated assault. In 2016, Diane Staudte and her daughter Rachel, from Springfield, Missouri, were both given life sentences after admitting to killing first her husband, Mark, and then her son, Shaun, with antifreeze. Next her elder daughter Sarah became seriously ill, for the same reason, but survived.
But it should be remembered that these crimes are very rare; it’s just that they do tend to grab the headlines. And victims chill out with antifreeze. Permanently.
Ah yes, thank you. In 2013, Jacqueline Patrick put antifreeze in her London-bus-driver husband Douglas’s Cherry Lambrini drink on Christmas Day; she and her daughter Katherine received a total of 18 years' sentence between them. She was caught because of a spelling mistake (‘dignaty‘) in a faked suicide note.
Yes, in a recent episode of the TV series Prison Break (season 5, episode 6, shown in May 2017, to be exact!), the main character, Michael Schofield, was stranded in a desert with a jeep but no water, and about 20 miles to walk to safety. And worse still, he'd been stabbed in the leg by a knife contaminated with antifreeze. He staggered home safely, but needed emergency medical treatment for poisoning, which included a blood transfusion!
If doctors diagnose ethylene glycol poisoning early enough, they can administer an antidote, such as ethanol. This binds to the alcohol dehydrogenase enzyme in the liver about 100 times more strongly than ethylene glycol does, so that by preventing the ethylene glycol from attaching itself to the liver, the kidneys have the chance to excrete the ethylene glycol unchanged and eliminate it harmlessly from the body. Fomepizole (whose name derived from the chemical name, 4-methylpyrazole) is a newer antidote, and works in the same way as ethanol.
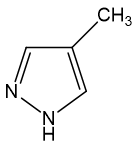 |
| Fomepizole |
Three different molecules are commonly used in antifreeze: ethylene glycol, diethylene glycol (DEG) and propylene glycol, especially the first one, which is responsible for most poisonings, accidental or otherwise.
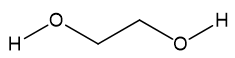 |
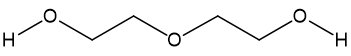 |
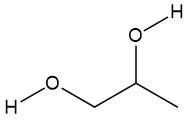 |
| Ethylene glycol | Diethylene glycol (DEG) | Propylene glycol |
Propylene glycol is much less toxic but diethylene glycol has been implicated in a whole range of poisonings. Like ethylene glycol, it has a very high boiling point (244°C). The most famous diethylene glycol poisoning case occurred in September-October 1937, when an American drug company wanted to market sulphanilamide (MOTM July 2011), a marvellous but somewhat insoluble antibiotic, in a liquid form. Their chief chemist, Harold Watkins, found that DEG was a very good solvent for sulphanilamide. He added red colouring and raspberry flavour, and the company sold it across the USA. Unfortunately he did no tests for toxicity. Around a hundred people, many of them children, died before it could be recalled. Watkins committed suicide. This led to the 1938 Federal Food, Drug, and Cosmetic Act, which gave the FDA authority to test all new drugs, and 25 years later this Act prevented thalidomide (MOTM July 2000) from being used in the USA.

Article from the New York Times in 1985 about the Austrian wine scandal.
|
In 1985, a scandal engulfed the Austrian wine industry. It was discovered that some wine manufacturers had been adding DEG to their sweet white wines to make them seem sweeter, more like the late-harvest Prädikatswein. Because the DEG concentrations were low, there were no human fatalities, though the Austrian wine industry received an almost-mortal blow. In 1996, 85 Haitian children died from adulterated cough-syrup containing DEG, and the same combination poisoned over 300 children in Bangladesh. DEG-containing expectorant has been blamed for the deaths of some 30 Indian children in 1998. Other cases have been reported from countries including Panama, South Africa, Nigeria and Argentina. Back in 2007 there were problems with some toothpastes imported from China, which turned out to contain DEG. The problem first surfaced in Panama but then spread. Counterfeit Colgate toothpaste containing DEG was found on sale in parts of the USA whilst counterfeit Sensodyne toothpaste turned up in England. At one point, the FDA banned all Chinese-made toothpaste from the USA. 119 cases of DEG poisoning occurred in Panama between June 1 and October 22, 2006, with 78 fatalities, due to a locally produced sugarless cough-syrup. On the other hand, another triol, glycerol (MOTM January 2018), is not toxic at all, being essential to the functioning of the human body. |
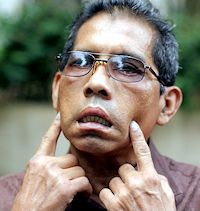 Ernesto Osorio from Panama had his face partly paralyzed after using cough-syrup contaminated with DEG. Photo: Ángel Franco/The New York Times |
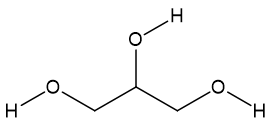 |
| Glycerol Propane-1,2,3-triol |
Small changes in structure can make a big difference in toxicity – just think of the difference between ethanol and the much more toxic methanol.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5611279]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5611279]