![]()
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Also available: HTML-only and VRML versions.
![]()
 "They couldn't hit an elephant at that distance"
"They couldn't hit an elephant at that distance" Who said that?
Who said that?
General John Sedgwick at the American Civil War battle of Spottsylvania, on May 9th 1864.
And?
They were his last words; seconds later, he was killed by a sniper!
Why would you want to hit an elephant anyway?
You might want to catch an elephant in the wild, or just immobilise a zoo elephant so it can have an operation.
How?
Generally they use dart guns. It's a bit trickier in the wild, as the elephants are less friendly, and usually part of a herd.
What sort of guns?
In the Kruger National Park (in South Africa), they use shotguns, firing darts based on .22 calibre blank cartridges.
What's in the darts?
Etorphine, which is a synthetic cousin of morphine and 1000 times more effective. A 4 mg dose will immobilise a 5000 kg African elephant; 1 mg will knock out a 2000 kg rhino.
If it does that to an elephant, what does it do to a human?
It's so toxic that it is usually dyed red, for ease of recognition. It was synthesised in the early 1960s by a research group at McFarlan-Smith and Co. in Edinburgh, led by Professor Kenneth Bentley. They were trying to make new non-steroidal anti-inflammatory drugs.

How did they discover its effect - animal tests?
You might say that. One day someone stirred the mid-morning cups of tea in the lab with a glass rod that, unknowingly, had been contaminated with etorphine. The chemists were laid out and nearly killed.
Bet they didn't do a risk assessment!
You're telling me! It's not the first time something like that happened. The German chemists who made the first nerve gases were guinea pigs unknowingly - they were trying to make insecticides at the time. Saccharin was discovered when someone forgot to wash his hands before leaving the lab and later licked his fingers. The important thing is that someone recognised these compounds could be useful; as Pasteur once remarked "Le hasard favorise l'esprit preparé" (Chance favours the prepared mind).
Where does etorphine act?
It is thought to work through the "opioid receptor". The simplest view of this is a one-receptor model, which has three main areas that bind morphine and its derivatives: an anionic site, of dimensions 8 Å by 6.5 Å, which binds the amine nitrogen (A) which can become positively charged; a cavity that accommodates the projecting piperidine ring (B); and a flat surface that binds the benzene ring (C) by Van der Waals' forces. It is also thought that there is a lipophilic pocket into which the C(OH)MePr sidechain present in etorphine fits, causing its increased activity in comparison with morphine, which does not have a group in this position.
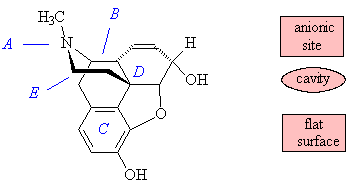
The rule for morphinoid activity has been summarised in a simplified form as "any structure that has a phenyl group attached to a quaternary carbon that in turn is two atoms distant from an amine nitrogen". In general, a number of features have to be present for the opioid analgesic to be active, including the tertiary nitrogen with a small alkyl group (A); the presence of a piperidine ring (B); a quaternary carbon (D) and a benzene ring (C) - or species with the same shape -attached to it; and a C2 spacer (E), part of the piperidine ring, between the tertiary nitrogen (A) and the quaternary carbon (D). A more sophisticated view is that there are three receptors, mu (m); kappa (k) and delta (d), that morphine-like molecules can bind to; different receptors bind different agonists better.
Anything else about etorphine?
Scientists at BAT (British American Tobacco) once debated adding it to tobacco as it might create an addictive craving for it. Some people call it "elephant juice"; it has been used to dope racehorses.
Bibliography
![]()