![]()
Ferric Chloride
aka Iron(III) Chloride
Not just for etching copper
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month October 2016
Also available: HTML version.
![]()
 |
Ferric Chloride
|
A lot of people still refer to it as ferric chloride, and this links with its origins, with the Latin ferrum or the French fer.
Transition metals often have two principal oxidation states. Traditionally, -ous is a suffix used to mark the lower oxidation state and –ic for the higher one. Thus, ferrous means iron (II) and ferric means iron (III). Similarly cobaltous means cobalt (II) and cobaltic means cobalt (III); cuprous means copper (I) and cupric means copper(II); and aurous means gold (I) and auric means gold (III).
Like Auric Goldfinger?Indeed. Ian Fleming obviously knew more chemistry than you would think. |
|
If you react iron (e.g. steel wool) with gaseous HCl, you get FeCl2. If, instead, you use chlorine gas, which is a stronger oxidising agent, you get FeCl3.
2Fe(s) + 3Cl2(g) 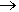 2FeCl3 (g)
2FeCl3 (g)
At the temperature of the reaction (> 200ºC), the iron chloride vapourises out of the hot zone and is collected as a brown-black sublimate.
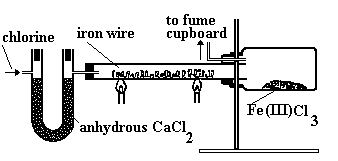

Apparatus used to make anhydrous FeCl3 and a photo of it as a brown-black powder.
There are other routes; for example it can be made from refluxing Fe2O3 with CCl4 as the chlorinating agent, and by dehydrating FeCl3.6H2O with SOCl2, thionyl chloride. If you try dissolving Fe2O3 in hydrochloric acid, you get the yellow-black hydrate, FeCl3.6H2O.
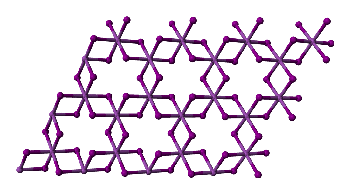 And the structure of FeCl3?
And the structure of FeCl3?In the solid state, FeCl3 adopts the same structure as BiI3; each iron is octahedrally coordinated by six chlorines, as shown on the right.
It is volatile above around 300ºC, and the vapour phase then contains substantial amounts of Fe2Cl6 molecules. At higher temperatures, around 600ºC, the monomeric form of FeCl3 is present.
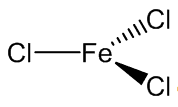 |
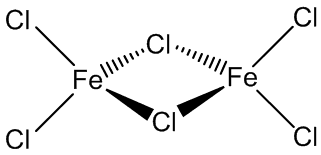 |
| FeCl3 | Fe2Cl6 |
_chloride_hexahydrate.jpg) How about when you dissolve it in water?
How about when you dissolve it in water?That gets complicated. A yellow-brown hydrate FeCl3.6H2O can be crystallised from solution, as shown in the photo, right. In the solid state, this is trans-[Fe(H2O)4Cl2]+ Cl-.2H2O, containing trans-[Fe(H2O)4Cl2]+ ions.
Studies on aqueous solutions of FeCl3 by both Extended X-Ray Absorption Fine Structure (EXAFS) and X-ray Absorption Near Edge Structure (XANES) identified several species: [Fe(H2O)6]3+, [FeCl(H2O)5]2+ and [FeCl2(H2O)4]+, at different solution concentrations.
![trans-[Fe(H2O)4Cl2]+ trans-[Fe(H2O)4Cl2]+](trans-fecl2h204.gif)
trans-[Fe(H2O)4Cl2]+
Some of the chloride ligands surrounding the iron get replaced by water molecules. This affects the splittings between the energy levels, as water produces bigger splittings than chloride, so this affects the energy of the electronic transitions, just as with cobalt chloride (MOTM June 2016). There are other factors too.
Under certain conditions, other hydrates can be isolated from solution. Back in 1892 it was reported that compounds with the composition FeCl3.3½.5H2O, FeCl3.2½H2O and FeCl3.2H2O could all be isolated. FeCl3.3½H2O is thought to be cis-[Fe(H2O)4Cl2]+ [FeCl4]-.3H2O, while FeCl3.2½H2O is known to have the structure cis-[Fe(H2O)4Cl2]+ [FeCl4]-.H2O.
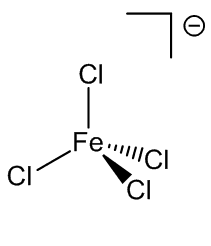
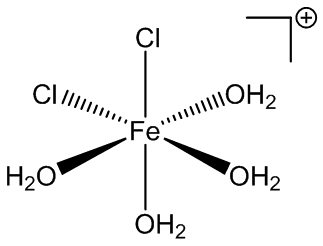
FeCl4-and cis-FeCl2(H2O)4+ ions in FeCl3.2½H2O.
FeCl3.2H2O is believed to be trans-[Fe(H2O)4Cl2]+ [FeCl4]- although FeCl3.2H2O molecules have been encapsulated by a crown ether, 15-crown-5.
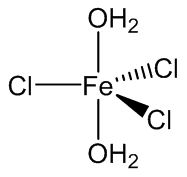
FeCl3(H2O)2 molecules trapped by 15-crown-5.
In addition, the two isomers of [FeCl3(H2O)3] have been isolated when co-crystallised with organic cation-chloride salts.
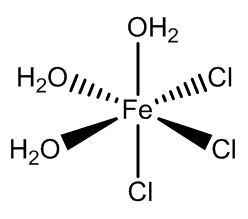 |
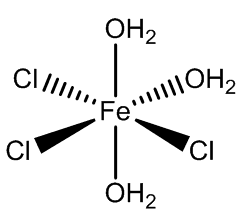 |
fac-FeCl3(H2O)3 |
mer-FeCl3(H2O)3 |
Although anionic species are not usually found in aqueous solutions of FeCl3, they are formed when hydrochloric acid is added, and a number of these have been characterised.
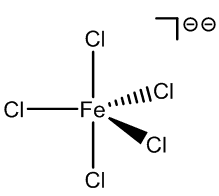 |
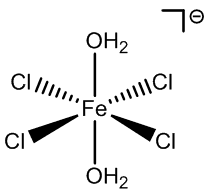 |
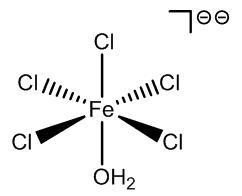 |
FeCl52- |
FeCl4(H2O)22- |
FeCl5(H2O)- |
There’s actually only a small difference in stability between the different isomers. Small factors – like intermolecular forces, e.g. hydrogen bonds to a water molecule – can tip the balance one way or the other.
Yes, thousands of them. The diagram below shows a variety of the structural possibilities.
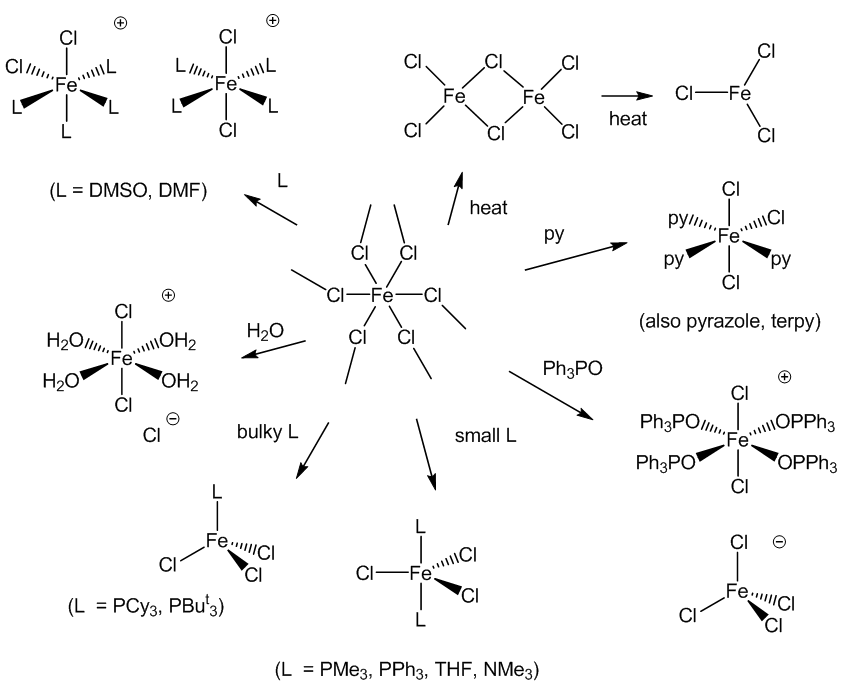
There are some general principles. Most complexes are octahedral. Some result from adding other ligands onto the FeCl3 unit – the more bulky the ligand is, the fewer can be fitted round the iron. In some cases it is more complicated – quite a few complexes with the simple ‘FeL2Cl3’ formula are not what they appear to be – something called ‘self’-ionisation’ occurs with the formation of ions, [FeL4Cl2]+ and [FeCl4]-. You can see this happening with Ph3PO, DMSO (Me2SO) and DMF (HCONMe2), for example. Then there is the possibility of isomerisation. Many FeL3Cl3 complexes (L = py, pyridine; pyrazole; 1/3 terpyridyl) are the mer-isomer, as shown.
Many factors have to be taken into account to explain the final result, which is why it seems complicated. But rewarding in its richness.
In the laboratory, anhydrous FeCl3 can be used as an alternative to anhydrous AlCl3 in Friedel-Crafts reactions, like the alkylation or acylation of aromatic rings. Being a small, highly charged metal, it polarises reagents like halogenoalkanes (alkyl halides), which generates carbocations (carbenium ions) that then attack benzene rings, as in the synthesis here.
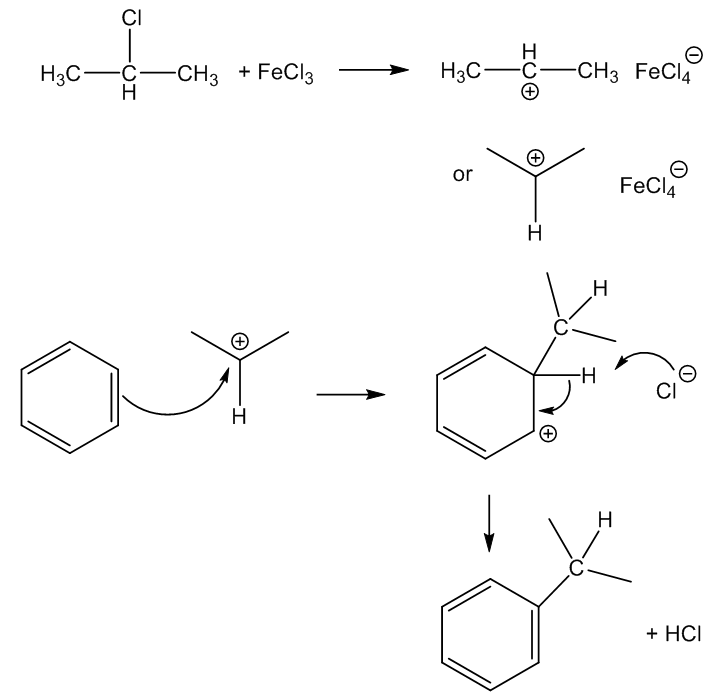
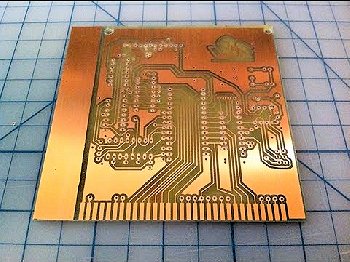 Is that it?
Is that it?No, in the world out there, it is used in etching copper to make printed circuit boards like the one shown on the right.
The Fe3+ ion is small and has a high charge, which makes it very polarising. That draws the electron pairs in the Fe-O bonds towards the iron, in turn polarising and weakening the O-H bonds, favouring their ionisation.
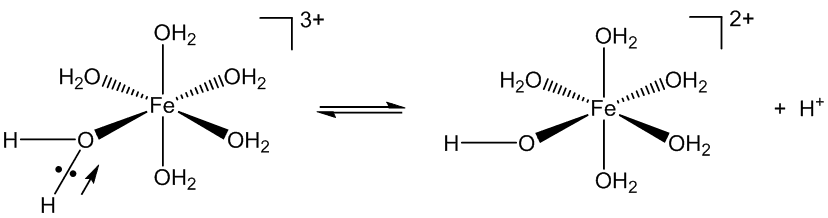
 But that is not why it is used in etching.
But that is not why it is used in etching.
The use of FeCl3 solutions in etching copper printed-circuit-boards (as in the photo, right) uses a different piece of chemistry, redox, rather than acid-base, and the presence of chloride is important.
Because Fe3+ is quite strongly oxidising, it strips electrons from copper atoms. In an overview, it forms Cu2+ ions and itself is reduced to Fe2+.
2 Fe3+(aq) + Cu (s) ⇌ 2 Fe2+(aq) + Cu2+(aq)
In more detail, oxidation at the surface forms a Cu(I) species, which diffuses away and is oxidised to copper(II) in the solution.
Cu (s) + Fe3+(aq) + 3Cl- (aq)  CuCl32- (aq) + Fe2+(aq)
CuCl32- (aq) + Fe2+(aq)
CuCl32- (aq) + Fe2+(aq)  Cu2+(aq) + Fe2+(aq) + 3Cl- (aq)
Cu2+(aq) + Fe2+(aq) + 3Cl- (aq)
By forming soluble complexes with copper(II), chloride prevents formation of insoluble copper(I) compounds, which would prevent further attack at the copper surface. Iron chloro complexes are also involved.
 One more thing. Do they use ferric chloride in Etch-A-Sketch?
One more thing. Do they use ferric chloride in Etch-A-Sketch?No.
![]()
![]()