IRON-MINERAL CORE
Note: Please use the scroll bar to see the entire
link.
Introduction
In this experiment, the ferritin contains an iron-mineral core
[FeO(OH)]8[FeO(H2PO4)]. The
iron-mineral core is attached to the inside of the protein wall
(as depicted in Scheme I of the main section of the tutorial). It
is bound covalently to the carboxylate sidechains on the protein
wall. The protein is capable of storing as many as 4500 iron
atoms in its interior, giving a concentration equivalent to 0.25M
Fe inside the protein. (Recall, at pH=7, [Fe3+] = 1018
M due to formation of insoluble hydroxides.)
Questions: In your experiment, what amount of iron did the
ferritin sample contain? Was the sample saturated?
Molecular Model of the Ferritin Protein with Mineral Core
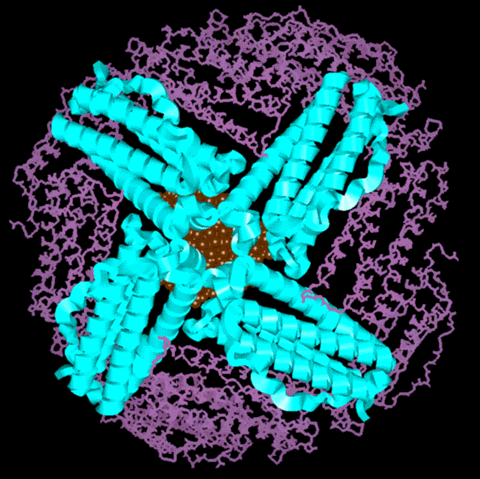 |
Figure 1:
This is a molecular model of the ferritin protein with
a representation of the iron-mineral core. The view looks
down the 4-fold channel at the mineral core. The subunits
that comprise the 4-fold channel are represented as
ribbons and the ribbons have the same color code as the
ribbons in Figure 1 in the main section of the tutorial.
The rest of the subunits are shown in the stick
representation. The iron-mineral core is depicted as
rust-colored. This model shows only half of the ferritin
shell. Recall that the mineral core is connected to the
protein shell with covalent bonds to carboxylate
residues. However, for a clearer view of the
three-dimensionality, the mineral core is not shown to be
connected to the protein shell in this picture.
|
Model Compound for Iron-mineral Core
Until recently, it was thought that all ferritin cores were
microcrystalline and identical. However, ferritin cores from a
variety of sources have now been studied using a variety of
experimental techniques (i.e., x-ray absorption spectroscopy,
Mossbauer spectroscopy, and high-resolution electron microscopy)
and a number of variations in the degree of structural and
magnetic ordering and level of hydration has been shown. One
simplified model compound of the iron-mineral core that has been
developed is [Fe12O2(OCH3)18(O2CCH3)6(CH3OH)4.67].
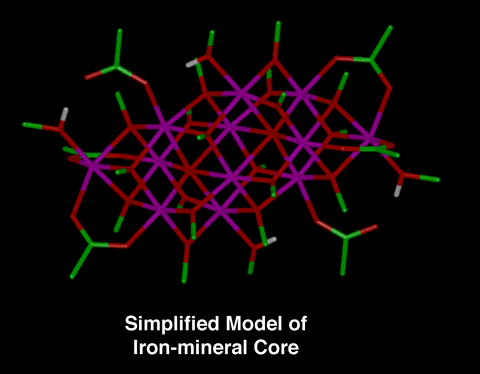 |
Figure 1:
This is a stick representation of the simplified model
compound for the iron-mineral core [Fe12O2(OCH3)18(O2CCH3)6(CH3OH)4.67].
The reference for this model compound is: K.L. Taft, et.
al., Science, 259, 1302 (1993). "A
Mixed-Valent Polyiron Oxo Complex that Models the
Biomineralization of the Ferritin Core."
Note: The carbon atoms are green, the hydrogens are
white, the iron atoms are magenta, and the oxygen atoms
are red in the stick representation.
|
- Please list the 3 major differences between the model
compound and the actual mineral core.
- Recall that the iron-mineral core is not freely
floating within the protein shell. The core binds to the
carboxylate sidechains on the protein wall. What type of
bond is formed (covalent or ionic)?
Return To
Iron-removal Process Section of Tutorial
Return To Compound-Structure
Index

