 The smell of filberts?
The smell of filberts?
Yes, though in many places they are known as hazelnuts (Corylus maxima and Corylus avellana), the fruit of the hazeltree (photo, right). And the molecule was only identified in hazelnuts in 1989. Hazelnuts are used as a flavouring in cakes, famously Viennese hazelnut torte and Kiev cake; confectionary (pralines) and chocolates; liqueurs; and other food products (like Nutella).
 |
 |
 |
Hazelnut prlaines | Kiev cake | Nutella |
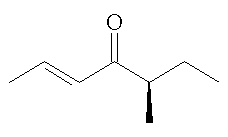
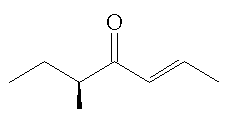
Filbertone is shown as enantiomers, do they both occur in nature?
In nature, there is a slight excess of the (S)-isomer, the amount varies depending upon the source of the filberts. The enantiomeric excess in the extracted filbertone is greater under mild extraction conditions.
 |
 |
(-)-(E,R)-filbertone |
(+)-(E,S)-filbertone |
|
|
Can you tell the isomers apart?
They have been separated by chromatography using a chiral column. The two mirror-image isomers (enantiomers) have significantly different smells. Both smell of "hazelnuts", but the (+)-(E,S)-isomer has a more fatty smell than the (-)-(E,R)-isomer, which has notes of butter and chocolate, as well as having a stronger impact, with a 10 times greater odour threshold.
How do you make it?
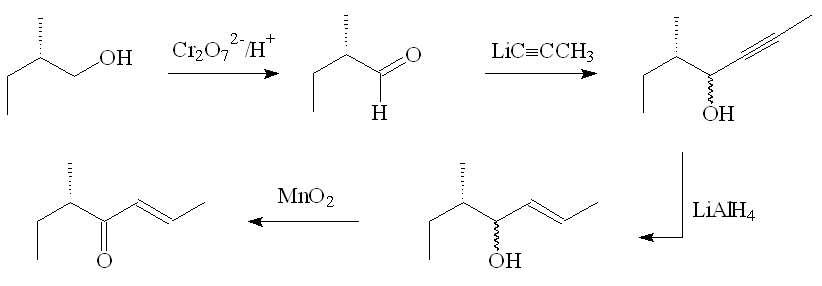
In the original laboratory synthesis, (+)-(E,5S)-filbertone was prepared starting from (S)-(-)-2-methylbutan-1-ol. First it was oxidised to the corresponding aldehyde, which underwent nucleophilic attack at the carbonyl carbon by propynyl lithium, forming a mixture of the isomers (4R,5S)-5-methylhept-2-yn-4-ol and (4S,5S)-5-methylhept-2-yn-4-ol. Reduction with LiAlH4 proceeded stereospecifically at the triple bond to give (E,4R,5S)-5-methylhept-2-en-4-ol and (E,4S,5S)-5-methylhept-2-en-4-ol, which on oxidation with MnO2 formed just (E,5S)-5-methylhept-2-en-4-one.
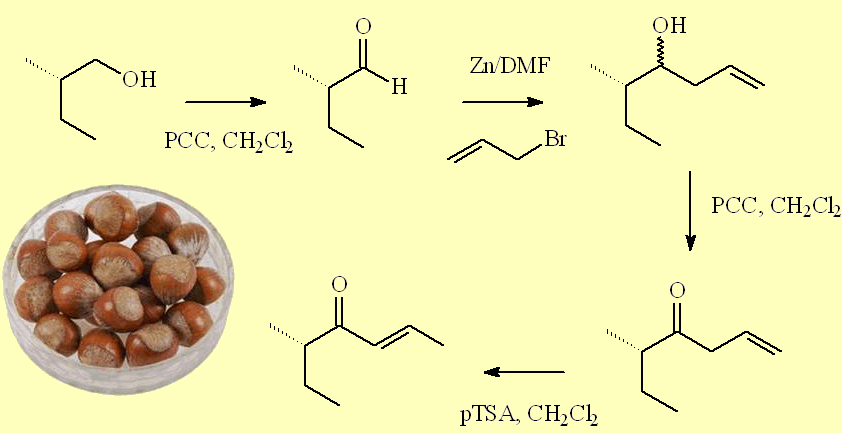
A second route uses oxidation in the first step, with pyridinium chlorochromate oxidant, but introduces the additional chain in a different way, by a coupling with allyl bromide. The alcohol product was oxidised using PCC again to afford (5S)-5-methylhept-1-en-4-one, which was isomerised on treatment with p-toluenesulfonic acid.
In a modification of the original route, NaOCl was used as the stoichiometric oxidant in the first step, with 2,2,6,6-tetramethylpiperidinyloxyl (TEMPO) catalyst. Yields were improved by better work-up procedures.
Is filbertone the only molecule responsible for the smell of hazelnuts?
Not at all. A recent paper on Italian hazelnuts (2010) identified 37 odour-active compounds in the raw nuts. Important ones include three substituted pyrazines, especially 2-methoxy-3-isopropylpyrazine and 2-methoxy-3,5-dimethylpyrazine, as well as 5-methyl-4-heptanone and ethyl 2-methylbutanoate, in addition to 5-methyl-(E)-2-hepten-4-one.
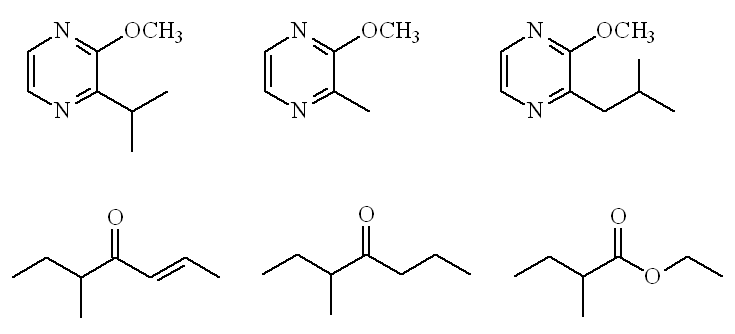
Raw hazelnut odourants
Rather more (46) aroma-active compounds were detected in roasted nuts, with some additional new molecules present, notably unsaturated aldehydes (Z)-2-octenal , (Z)-2-nonenal, (Z)-2-decenal and (E,E)-2,4-decadienal, 2-thienylthiol, 2-acetyl-1-pyrroline and 2-propionyl-1-pyrroline. |
 |
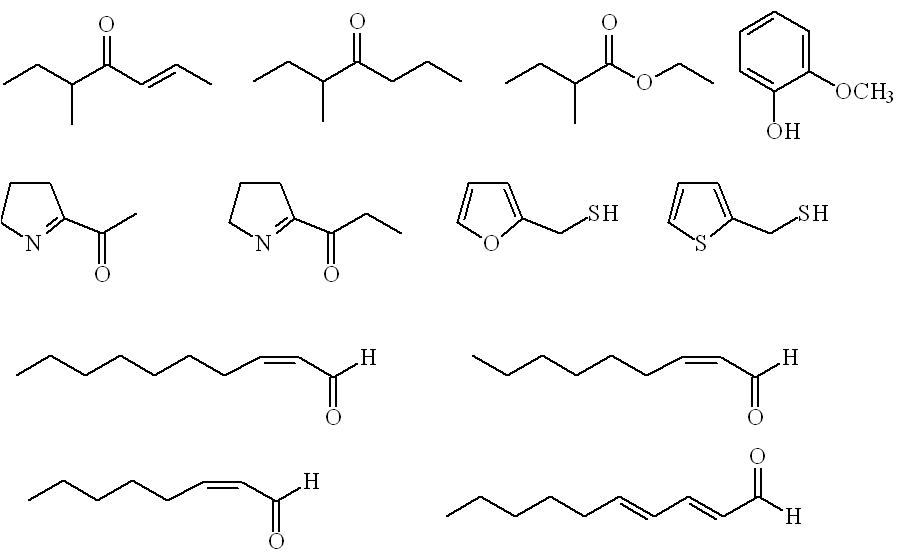
Roast hazelnut odourants
And what's this about using Hazelnut oil to adulterate olive oil?
It's true. Olive oil is a cornerstone of the Mediterranean diet. It's more expensive than hazelnut oil, so unscrupulous people adulterate olive oil with cheaper hazelnut oil, as small amounts can be hard to detect, though GC-MS has been successfully used. |

The Abbey at Tournus
|
And why are they called filberts?
They are linked with St. Philibert, the 7th-century monk who founded several monasteries in France, and whose feast day of August 20th coincides with the nutting season. He was buried at Noirmoutier, on the western coast of France, but the 9th-century Viking attacks caused the monks to move. They eventually settled at Tournus in the Rhone valley, where the great abbey church of St. Philibert still holds his relics. |
The casket (or reliquary) holding the relics of St Philibert.
|

Bibliography
General
- Chapman and Hall Combined Chemical Dictionary compound code numbers: - JJR47-D; JZS08-F, JZS09-G.
Syntheses
- J. Jauch, D. Schmalzing, V. Schurig, R. Emberger, R. Hopp, M. Kopsel, W. Silberzah and P.Werkhoff, Angew. Chem. Int. Ed., (1989), 28, 1022-1023 (isolation and synthesis)
- J. Jauch, H. Czesla and V. Schurig, Tetrahedron, (1999) 55, 9787-9792 (improved synth.)
- P.H.G. Zarbin, M. Yonashiro and W. Perissini, J. Braz. Chem. Soc., (1998), 9, 583-585. (synth.)
Hazelnut odorants
- http://www.bojensen.net/EssentialOilsEng/EssentialOils13/EssentialOils13.htm
- C. Alasalvar, A.Z. Odabasi, N. Demir, M.Ö. Balaban, F. Shahidi and K.R. Cadwallader, J. Food Sci., (2004), 69, 99-106 (volatiles in Turkish varieties).
- K. R. Cadwallader and S. Puangpraphant, in Tree Nuts. Composition, Phytochemicals, and Health Effects, F. Shahidi and C. Alasalvar, eds., CRC Press, Boca Raton, 2008, pp 113-115.
- A. Burdack-Freitag and P. Schieberle, J. Agric. Food Chem., (2010), 58, 6351-6359.
The enantiomers and their separation
- J. Jauch, D. Schmalzing, V. Schurig, R. Emberger, R. Hopp, M. Kopsel, W. Silberzah and P.Werkhoff, Angew. Chem. Int. Ed., (1989), 28, 1022-1023 (natural isomers)
- M. Guntert, R. Emberger, R. Hopp, M. Kopsel, W. Silberzahn and P. Werkhoff, Z. Lebens. Unters. Forsch., (1991), 192, 108-110. (smell of the isomers)
- G. P. Blanch and J. Jauch, J. Agric. Food Chem., (1998), 46, 4283-4286 (separation of enantiomers)
- M. L. Ruiz del Castillo, G. Flores, M. Herraiz and G. P. Blanch, J. Agric. Food Chem., (2003), 51, 2496-2500. (separation of enantiomers)
- G. P. Blanch and M. L. R. del Castillo, J. Sep. Sci., (2006), 29, 691 - 694 (separation of enantiomers)
- http://www.leffingwell.com/chirality/filbertone.htm (smell of the isomers)
Roasted hazelnuts
- C. Alasalvar, F. Shahidi and K. R. Cadwallader, J. Agric. Food Chem.,(2003), 51, 5067-5072.
- C. Cordero, E. Liberto, C. Bicchi, P.Rubiolo, P. Schiberle, S. E. Reichenbach and Q. Tao, J. Chromat., (2010), 1217, 5848-5858.
- A. Burdack-Freitag and P. Schieberle, J. Agric. Food Chem., (2010), 58, 6351-6359.
Hazelnut oil in adulterated olive oils
- M.M. Caja, M.L. Ruiz del Castillo, M. Herraiz and G.P. Blanch, J. Am. Oil Chem. Soc, (1999), 76, 1027 - 1030
- G. P. Blanch, M. M.Caja, M. L. Ruiz del Castillo and M. Herraiz, Eur. Food Res. Technol., (1999), 210, 139-143
- J. L. P. Pavón, M. N. Sánchez, M. E. F. Laespada and B. M. Cordero, Anal . Bioanal. Chem., (2009), 394, 1463-1470
Tournus and St Philbert


 Back to Molecule of the Month page.
Back to Molecule of the Month page.
![]()
![]()
![]()
![]()

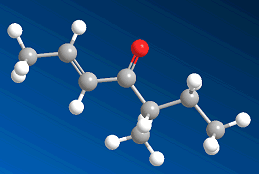


 The smell of filberts?
The smell of filberts?









![]()
![]()