![]()
Galanthamine
(Commercial names: Reminyl, Nivalyn, and Razadyne)
The anti-Alzheimers' drug derived from snowdrops
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month November 2012
Also available: JSMol version.
![]()
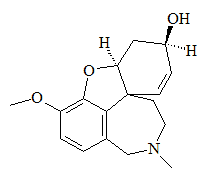
Galanthamine(Commercial names: Reminyl, Nivalyn, and Razadyne)The anti-Alzheimers' drug derived from snowdrops
Simon Cotton
Molecule of the Month November 2012
|
 |
What's the story?
I thought that Homer's wife was Marge, not Moly?That's the wrong Homer! We are talking about the great Greek poet, who lived around the 8th-century BC, author of both the Iliad and the Odyssey. In the Odyssey, the story is concerned with the ten years it took Odysseus (Ulysses) to return from Troy to his home in Ithaca, after the end of the Trojan War. In Book 10 of the Odyssey, Odysseus and his crew arrive at the island of Circe, who is both a witch and a goddess. She fed half the crew a meal that had been drugged, and turned them into swine. Odysseus had been warned in advance by Hermes and given a herb called Moly which stopped Circe's spell working, so that he was unaffected and able to resist her charms and spells. The herb is described as having a black root but a white flower. Some think that this could be a description of a snowdrop, such as the common snowdrop (Galanthus nivalis, photo: below, right), which is found across a very wide area of Europe, as far east as Greece and parts of Turkey. |
|
 And next?
And next?Fast forward over 2500 years. According to one story, a Bulgarian pharmacologist was visiting a rural area around 1950 when he saw people rubbing snowdrops on their foreheads to ease headaches, and his curiosity was aroused. It is certainly true that in 1952 Russian scientists isolated galanthamine from Caucasian snowdrops (Galanthus woronowii) and within a few years it was being used in anaesthesiology as a curare reversal agent and also in the treatment of poliomyelitis. Along with other alkaloids, it has been found in a range of related Amaryllidaceae plants including Leucojum aestivum (Summer snowflake) and Narcissus pseudonarcissus (wild daffodil), as well as Lycoris radiata (Red Spider Lily).
Yes, but because of increased demand, not enough plant-derived galanthamine is available, so chemical synthesis is increasingly being looked at as an option.
It is a selective, competitive and reversible inhibitor of acetylcholinesterase. It also modulates neural nicotinic receptors to increase acetylcholine (ACh) release.
By stopping the enzyme acetylcholinesterase (AChE) from breaking down acetylcholine in the brain, it maintains acetylcholine levels. Acetylcholine is a key neurotransmitter, a molecule that transmits nerve messages when they are released from neurons to travel across a synaptic cleft, where they bind to receptors. Alzheimers' disease is believed to be associated with a degeneration of the neurons which links acetylcholine and a decline in these messengers with memory loss.
Alzheimer's disease affects the brain and is associated with a decrease in acetylcholine levels; because it can cross the blood-brain barrier, galanthamine can get into peoples' brains and stave off decline in ACh levels, thus maintaining brain function and thinking levels. It is effective in patients with mild to moderate Alzheimers'.
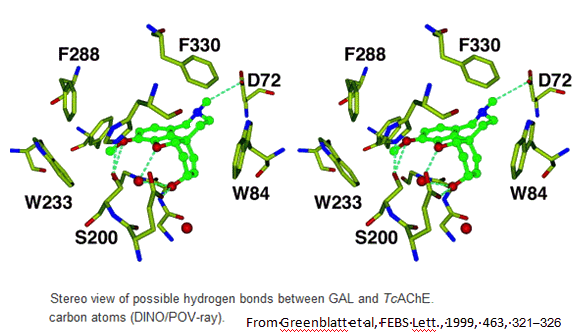
In 1999 scientists first reported the crystal structure of a complex of galanthamine with Torpedo californica acetylcholinesterase. Galanthamine sits in the active site of the AChE, occupying both the acyl-binding pocket and the choline-binding part. It is involved in a number of interactions which are weak individually, but which collectively make galanthamine bind quite well to the AChE. The OH group of the galanthamine hydrogen-bonds with a glutamic acid group of the enzyme and also interacts with two water molecules. The tertiary-amine group of the galanthamine is protonated under the isolation conditions, and the N-H group hydrogen bonds with an aspartic acid group, and also with a water molecule. The methoxy group is held in the acyl-binding pocket of the AChE by a number of interactions - the hydrogens of the methyl group are involved in non-bonded σ-π interactions with three different phenylalanine residues, whilst the oxygen hydrogen-bonds to both a serine group and to a water molecule. Finally, the cyclohexene ring of galanthamine forms a π-π interaction with the indole ring of a tryptophan in the enzyme.
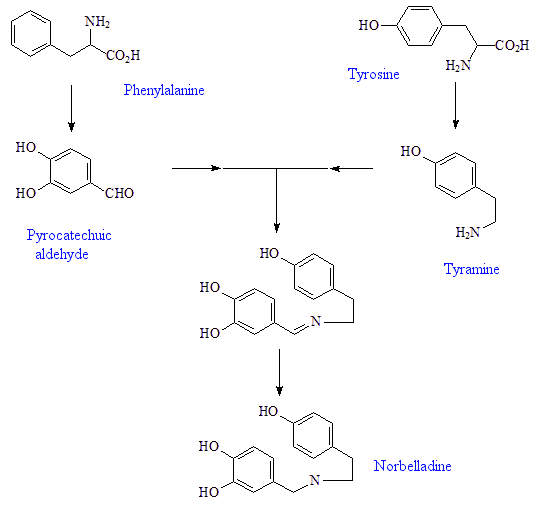
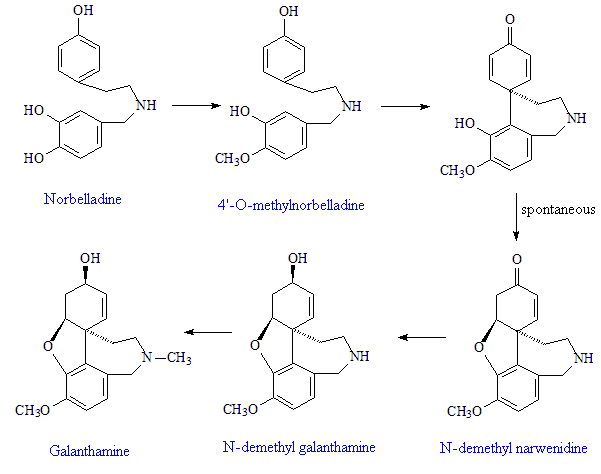
The plants start with the amino-acid phenylalanine, which they convert into pyrocatechuic aldehyde, and tyrosine, which they convert into tyramine. These condense together to form Schiff-base precursors to the norbelladine, a precursor to 4'-O-methylnorbelladine, which undergoes intramolecular oxidative phenol coupling affording a dienone. This spontaneously cyclizes to demethylnarwenidine, then reduces to demethylgalanthamine, which on N-methylation affords galanthamine.
 Does galanthamine have any other applications?
Does galanthamine have any other applications?There has been a certain fashion for using galanthamine non-clinically as it is reported to enhance dreams. More interestingly, it has been reported to improve sustained attention in chronic cocaine users.
We don't know that it won't turn out to be useful to other people. But in any case, more and more people are living to an old age, and galanthamine could turn out to help millions. J.M. Barrie (the playwright and author of Peter Pan, photo, right) once said that God gave us memories "so that we might have roses in December". Sadly, this is not true for all elderly people, so anything that helps us keep memories is a good thing.
![]()
Chapman and Hall Combined Chemical Dictionary compound code number: - CDN46-T
IUPAC name: (4aS,6R,8aS)-5,6,9,10,11,12-hexahydro-3-methoxy-11-methyl-4aH-[1]benzofuro[3a,3,2-ef] [2] benzazepin-6-ol or 4a,5,9,10,11,12-Hexahydro-3-methoxy-11-methyl-6H-benzofuro[3a,3,2-ef][2]benzazepin-6-ol.
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255767]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255767]