Organization of GalCer in the plasma membrane
: rafts
Membrane lipids are amphiphilic
molecules. This means that they are formed of two parts, one hydrophobic
(apolar) and the other hydrophilic (polar). The polar part interacts with
water, whereas the apolar part has to find an apolar phase to avoid contact
with water. In other words,
membrane lipids have no other choice than to self-organize into supramolecular
complexes in which only their polar part interacts with water. Biological
membranes are formed by a bilayer of lipids, with a hydrophobic core formed by
the hydrocarbon chains of lipids. In the very popular fluid mosaic model of
biological membranes, lipids form a homogeneous two-dimensional solvent phase
for membrane proteins. Yet membrane lipids comprise several hundreds ofdistinct molecules that exist in different physical states controlled by
several physicochemical parameters such as the temperature, presence of
cholesterol and chemical nature of the hydrocarbon chains.
Thus, biological membranes are probably
better described as a Ômosaic of lipid domainsÕ rather than a homogeneous fluid
mosaic. Membrane cholesterol, for instance, is unevenly distributed into
cholesterol-rich and cholesterol-poor domains, consistent with the notion that
specialized lipid domains with specific biochemical composition and
physicochemical properties do exist in membranes. Among these domains, those
containing sphingolipids and cholesterol, referred to as lipid rafts or
caveolae (when associated with the integral membrane protein caveolin), have
been extensively studied.
Why do sphingolipids and cholesterol
self-associate and segregate into specific membrane domains? The answer to this
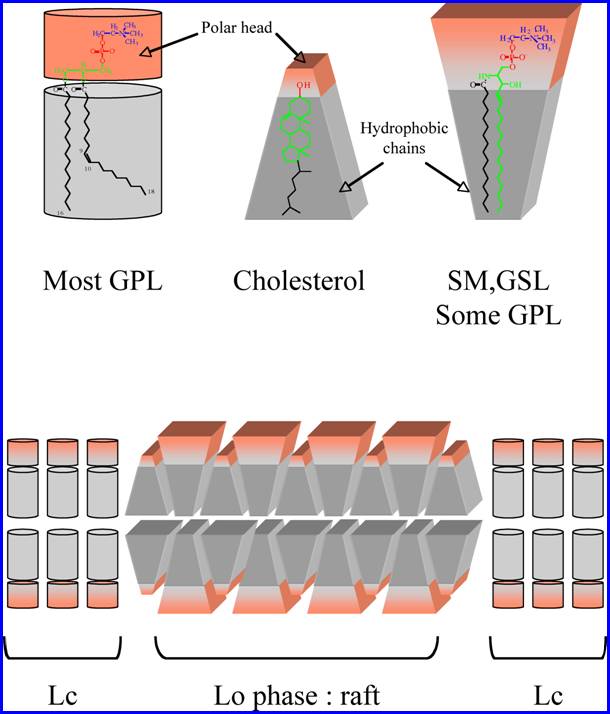
question may be given by the biochemical structure of membrane lipids. Glycerophospholipids
such as phosphatidylcholine (PC) are rich in kinked unsaturated acyl chains
(with C=C double bonds in the cis configuration), whereas the hydrophobic part
of sphingolipids such as sphingomyelin or glycosphingolipids (GSL) contain a
saturated acyl chain and sphingosine. Introducing a C=C double bond of cis
geometric configuration results in a bending of the chain. This change from the
linearity impairs the tight packing of lipid chains, so that
glycerophospholipids have more mobile hydrophobic chains than sphingolipids.
Since the mobility of the hydrophobic lipid anchor in the apolar phase of the
membrane interferes with the packing capacity of lipid molecules, the energy
required to separate two adjacent sphingolipid molecules is significantly higher
than for glycerophospholipids. This energy can be quantified by measuring the
temperature required to induce the solidÐliquid phase transition of a lipid,
i.e. the melting temperature (Tm). The Tm of PC is as low as
GalCer, like most sphingolipids, is
found in the external leaflet of the plasma membrane within lipid rafts. This
unique localization has important consequences for the biological functions of
GalCer.