Therapeutic approaches
The important contribution of cell
surface carbohydrates to microbial adhesion onto mucosal surfaces has motivated
the design of synthetic glycoconjugates as potential antimicrobial agents. An
immediate approach to mimick natural antibacterial strategies is to use the
oligosaccharides that are found at concentrations as high as millimolar in
human milk. Indeed, milk oligosaccharides are responsible for the protective
effects of non-immunoglobulin fractions of human milk. However, the hydrophobic
part of GSL receptors and, at least in some cases, cholesterol, have a profound
impact on the orientation of the oligosaccharide moiety, so that free
oligosaccharides have often a reduced affinity for ligand compared to the whole
membrane-anchored GSL. Moreover, GSLreceptors are concentrated in lipid rafts,
so that the free oligosaccharide may not been able to reach the local
concentration and/or the active conformation of the carbohydrate binding domain
of intact GSL receptors. For these reasons, two main strategies have been
developed to increase the antimicrobial activity of carbohydrate-based
candidate drugs. The first approach is to polymerize the oligosaccharide on a
chemical matrix to obtain a multivalent neoglyconjugate. The other approach is
to modify the structure of the hydrophobic part of GSL receptors with the aim
to obtain water-soluble analogs in which the conformation of the binding domain
of the analog is as close as possible to that of the GSL. Correspondingly,
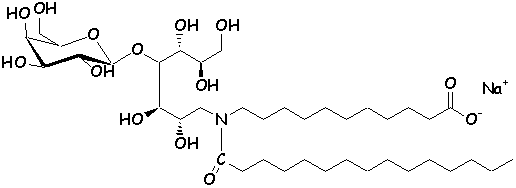
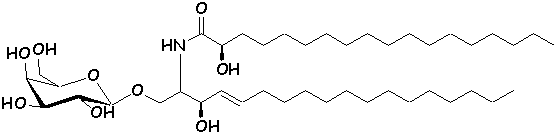
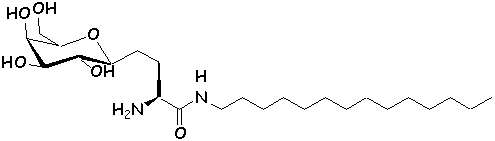
these analogs can be referred to as ‘glycolipidomimetics’ (16). Several synthetic analogs of GalCer have been
synthesized, with interesting anti-HIV activity in vitro (20-22).
The chemical structure of authentic GalCer (1) and synthetic analogs (2,
3) (23,
24) are shown below.
![]()
![]()
![]()