![]()
Geosmin
The smell of the countryside.
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Molecule of the Month August 2009
Also available: JSMol version.
![]()

GeosminThe smell of the countryside.
Simon Cotton
Molecule of the Month August 2009
|
 |
Because it is the molecule released by moist soil, or from soil that is freshly ploughed. It gets its name from the Greek for "earth odour".
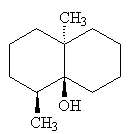 Geosmin |
 |
It is formed by microorganisms in the soil, particularly Streptomyces. Although the smell of geosmin was first identified in 1891, the isolation and structure of the geosmin molecule responsible for it was only reported in 1965, and its synthesis in 1968. It is even more recently that its biosynthesis has been understood.
Geosmin biosynthesis begins in farnesyldiphosphate (FPP), a key building block in sterol and sesquiterpene biosynthesis. An enzyme called germacradienol-geosmin synthase (GGS) contains two very similar catalytic domains. One domain converts FPP into germacradienol, which diffuses into the second domain close by, where the conversion to geosmin is completed.
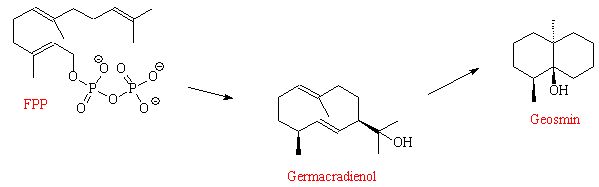
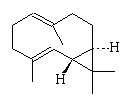
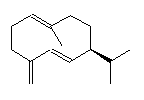
Intermediate compounds are involved, such as germacrene D, octalin and isolepidozene.
 |
 |
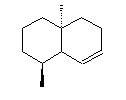 |
| Isolepidozene | Germacrene D | Octalin |
And only the (-) isomer of geosmin is formed.
The (+) isomer can be prepared in the laboratory, but tests show that the natural (-) form has a significantly stronger smell. On average, (-) geosmin has an 11![]() lower odour threshold than the (+) isomer, quoted as 0.0082-0.018 ppb (in water).
lower odour threshold than the (+) isomer, quoted as 0.0082-0.018 ppb (in water).
It is a colourless liquid, with a boiling point of 270°C.
Geosmin (and also 2-methylisoborneol, which also has an undesireable taste) is often responsible for unpleasant tastes in water supplies. Cyanobacteria (blue-green algae) and actinobacteria release geosmin when they die, and this can be absorbed by bottom-feeding freshwater fish. This is a particular problem in parts of the United States, giving off-flavours to fish (particularly catfish (photo, below), carp and mullet) and clams. Geosmin is made odourless by acid, so these fish are often eaten with lemon, leading to recipes like clam chowder with lemon zest, blackened Cajun catfish or grilled catfish with lemon juice.
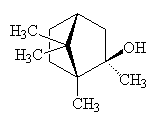 Methylisoborneol |
 Catfish and beetroot can both smell of geosmin |
 |
 Geosmin is a major contributor to the smell and flavour of beetroot, a variety of Beta vulgaris. Not everyone likes the smell, and since geosmin levels vary between cultivars, selective breeding can produce varieties with a bland taste designed to appeal to mass markets.
Geosmin is a major contributor to the smell and flavour of beetroot, a variety of Beta vulgaris. Not everyone likes the smell, and since geosmin levels vary between cultivars, selective breeding can produce varieties with a bland taste designed to appeal to mass markets.
Geosmin and the related molecule dehydrogeosmin are responsible for the musty smell of some plants, especially certain cacti.
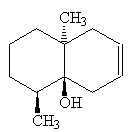 Dehydrogeosmin |
In recent years, notably 2002, French wine growers have been concerned about the presence of a rather musty smell in some young wines, which was quite distinct from the usual "musty cork" smell due to 2,4,6-trichloroanisole. Geosmin was identified as the culprit in these wines using gas chromatography-mass spectrometry, specifically the (-) isomer. Corks or the wood of barrels were excluded as the source of the geosmin, as the wines had been stored in stainless steel containers or bottles with metal stoppers. Geosmin was then detected in some grape juices prior to fermentation, and it transpired that geosmin was formed on rotten grapes through the combined action of both the Botyris cinerea and Penicillium expansum fungi (see photo, left).
Well, acid destroys it; it has also been found that photocatalysts based on titanium dioxide (TiO2) can also destroy water-borne geosmin.
 So geosmin is pretty useless?
So geosmin is pretty useless?Not if you are a camel in search of an oasis!
Professor Keith Chater, of the John Innes Centre in Norwich (UK), was a member of the team that in 2002 sequenced the genome (8000 genes) in Streptomyces coelicolor A3, a bacterium that produces many chemicals, including geosmin. In the following year he reported identifying a protein domain needed for biosynthesis of geosmin. He has suggested that camels can detect the smell of geosmin that had been released by Streptomyces miles away in wet ground, and track the geosmin to find an oasis; in return the camel could carry away and disperse the spores of the Streptomyces bacterium.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255047]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255047]