![]()
HYDROGEN PEROXIDE
Rocket fuel and bleached blondes...
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Molecule of the Month September 2006
Also available: JSMol version.
![]()

|
HYDROGEN PEROXIDERocket fuel and bleached blondes...
Simon Cotton
Molecule of the Month September 2006
|
 |
 Who was the first famous peroxide blonde?
Who was the first famous peroxide blonde?This was Jean Harlow (1911-1937). She was ash-blonde to begin with, but became a "platinum blonde" through regular use of hydrogen peroxide, ammonia and other chemicals, including Clorox bleach (based on sodium hypochlorite). Her film Platinum Blonde (1931) popularised the expression. She was followed by Marilyn Monroe (1926-1963), who starred in Gentlemen Prefer Blondes (1953) and others, up to Reese Witherspoon of Legally Blonde (2001). Not forgetting Debbie Harry, of Blondie.




By use of very slightly alkaline hydrogen peroxide solution. The hydrogen peroxide oxidises the melanin pigment inside the hairs to a colourless substance; the alkali makes the hair more permeable to the H2O2 by softening the cuticle, so the peroxide can reach the melanin.
It depends on what you mean by "unstable". It is stable with respect to the elements:
H2 (g) + O2 (g) ![]() H2O2(l) (ΔfH = -187.6 kJ mol-1, ΔfG = -118 kJ mol-1)
H2O2(l) (ΔfH = -187.6 kJ mol-1, ΔfG = -118 kJ mol-1)
However, its decomposition products, water and (di)oxygen gas are even more stable, so its decomposition is exothermic too:
H2O2(l) ![]() H2O (l) + 1/2 O2 (g) (ΔfH = -98.2 kJ mol-1)
H2O (l) + 1/2 O2 (g) (ΔfH = -98.2 kJ mol-1)
It is stable in the absence of catalysts, but since some glasses can speed its decomposition, it is often kept in bottles made of Teflon or polythene. Stabilisers are usually added to retard decomposition.
Many substances catalyse its decomposition, like transition metals and their compounds, such as MnO2; the enzyme catalase in the human liver does the important job of removing toxic H2O2 (a byproduct of oxidation reactions) from the body. Even dust can act as a catalyst. They do this, of course, by reducing the activation energy for its decomposition.
| Catalyst | Ea (kJ/mol) |
|---|---|
| None | 75 |
| MnO2 | 58 |
| Iodide ion | 56 |
| Pt (colloidal) | 49 |
| Catalase | 23 |
Concentrated H2O2 is very unstable, as we will see.
When it's pure, hydrogen peroxide is an almost colourless (very pale blue) substance that resembles water (and mixes with it in all proportions). It freezes at -0.41°C and boils at 150.2°C (extrapolated to 760 mm pressure). Its density is 1.44 g cm-3 in the liquid state at 25°C and 1.64 g cm-3 in the solid state at -4.5°C, so that, unlike ice, solid H2O2 sinks when placed in the corresponding liquid form. It has a skewed structure with a dihedral angle of 111.5° (gas phase), which minimises repulsion between the lone pairs and the O-H bond pairs. The dihedral angle is affected by hydrogen bonding; it is 90.2° in solid H2O2.
It is prepared commercially mainly by catalytic oxidation of 2-alkylanthroquinones.
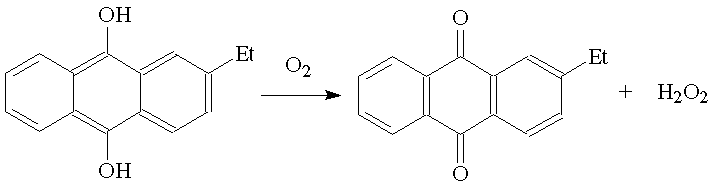
A more traditional route is the reaction of barium peroxide with sulphuric acid; the insoluble barium sulphate can be filtered off, leaving a solution of hydrogen peroxide.
BaO2 (s) + H2SO4 (aq) ![]() BaSO4 (s) + H2O2 (aq)
BaSO4 (s) + H2O2 (aq)
It is difficult to concentrate this solution by distillation (unless it is done at very low pressure), owing to decomposition of the peroxide; removal of water by evaporation in an air current is one option.
Around 2 million tons of hydrogen peroxide are made each year. About half of that is used to bleach wood pulp or paper. It is seen as an environmentally friendly alternative to chlorine-based bleaches. In domestic uses, it is used as a mixture with NaOH to bleach wooden surfaces. It is also used to bleach textiles.
Medically, hydrogen peroxide is used to disinfect skin, as it can kill bacteria by using its oxidising power. When it comes into contact with a cut, it fizzes as oxygen is released, probably due to the action of catalase in the blood. Dilute H2O2 solution (around 3%) is used as a mouthwash, and also for cleaning and disinfecting contact lenses. It also bleaches teeth.
Controversially, it has been suggested that use of hydrogen peroxide solution - for example in very low concentrations intravenously - can be used as a therapy for cancer ("oxygen therapy"). The American Cancer Society says that there is nothing to support this, and that it may well be harmful.
Definitely not. Hydrogen peroxide has found all sorts of uses, often in weapons. Starting with the bombardier beetle...
 What's that?
What's that?When assaulted, bombardier beetles (like the African bombardier beetle, Stenaptinus insignis, shown in the picture), spray a hot mixture of irritating p-benzoquinones and hydrogen peroxide from the end of their abdomen. They can aim this spray accurately in any direction. Usually in the wild they are aiming at predators like ants.
The bombardier beetle stores a mixture of the hydrogen peroxide and hydroquinones. At the moment that the defence is needed, the mixture is pumped into a reaction chamber, where it is mixed with catalysts (catalases and peroxidases) which catalyse the reaction decomposing the peroxide to oxygen. This oxidises the hydroquinones to quinones, very exothermically. The oxygen gas propels the hot spray out of the jet on the abdomen.
 Well, first there was the Messerschmitt 163 rocket aircraft ("Komet"). This wasn't a conventional jet aircraft, it was actually the only rocket aircraft to ever fly in operational service in WW2. It was fitted with a rocket motor that made it far faster than any other fighter in the air, but the fuel used made it somewhat "unsafe".
Well, first there was the Messerschmitt 163 rocket aircraft ("Komet"). This wasn't a conventional jet aircraft, it was actually the only rocket aircraft to ever fly in operational service in WW2. It was fitted with a rocket motor that made it far faster than any other fighter in the air, but the fuel used made it somewhat "unsafe".
The two fuels were "C Stoff" (57% methanol, 30% hydrazine, 13% water) and "T Stoff" (80% H2O2, 20% water), which were kept in separate tankers at opposite ends of the airfield and were always at least half a mile apart. It is said that any organic matter (including humans) could spontaneously combust in contact with the T Stoff. The fuels were loaded separately, with the aircraft and crew washed down carefully after the first fuelling, and again after the second one. A catalyst (Ca(MnO4)2 + K2CrO4) was mixed with some peroxide, generating a mixture of steam and oxygen that drove the pumps feeding the two fuels to the combustion chamber. The explosive reaction produced a mixture of hot gases, steam and nitrogen:
2 H2O2 (l) + N2H4 (l) ![]() 4 H2O (g) + N2 (g)
4 H2O (g) + N2 (g)
The plane would be doing 500 mph by the edge of the airfield, climb vertically at 440 mph, then approach American bombers at over 550 mph. No Allied fighter could keep pace with it, but the Me163's of JG 400 only shot down 9 bombers, and lost far more of their own in accidents. If a Me163 crash landed and somehow did not explode (they usually did), fuel lines could fracture, and the pilot would be dissolved alive by the T Stoff.
Hellmuth Walter, who designed the Me163, also designed a peroxide-powered U-boat (type XVIIB). It ran on very concentrated (95%) H2O2 ("Perhydrol") which on decomposition (using a catalyst) gave out superheated steam to drive a turbine; the oxygen formed was used for life support for the crew. Of the two boats built during the war, U1406 went to America, whilst the Royal Navy took over U1407, commissioning it as HMS Meteorite. It spent two years in experimental service. In the mid-1950s, the Royal Navy built two more peroxide-powered submarines, HMS Explorer and HMS Excalibur. Like U1406 and 1407, they were capable of over 25 knots submerged, but there were too many technical problems for them to be successful (like unstable 95% peroxide).
 And, sadly, there was the Kursk disaster. Kursk (photo, right) was the last of the huge Oscar-II class of nuclear attack submarines to be built for the Russian Navy (155 metres long, twice the length of a Boeing 747). It was launched in 1994.
And, sadly, there was the Kursk disaster. Kursk (photo, right) was the last of the huge Oscar-II class of nuclear attack submarines to be built for the Russian Navy (155 metres long, twice the length of a Boeing 747). It was launched in 1994.
On August 12th 2000, Kursk was taking part in a naval exercise off the Kola peninsula near Murmansk, when at 11.28 local time there was an explosion on board, equivalent to about 100 kg of TNT. This seems to have been caused by a leak of hydrogen peroxide fuel from a torpedo. This may have caused a fire, which detonated several other torpedoes, leading to a much bigger explosion 135 seconds later, reportedly with a force of around 7 tons of TNT (recorded by seismic stations 5000 km away, measuring 3.5 on the Richter scale). The submarine sank in 108 metres of water. The entire crew of 118 men was lost. The Royal Navy had abandoned the use of hydrogen peroxide as a torpedo fuel after the loss of HMS Sidon in Portland Harbour on June 16th 1955.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5248993]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5248993]