Coniine and the Hemlock Alkaloids
![]()
![]()
![]()
![]()
![]()
![]()
![]()
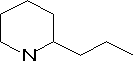
(+)-Coniine was fond to have the molecular formula C8H17N and had a specific rotation, [a]D, of +15.7o which implies the presence of at least one chiral centre. Hofmann (1884) obtained conyrine on distillation of (+)-coniine with zinc dust and this substance yielded pyridine-2-carboxylic acid (a-picolinic acid) on permanganate oxidation, which implies the presence of a pyridine ring with a side chain in the 2-position.
This molecular formula suggests that the heterocyclic ring is saturated indicating a piperidine rather than pyridine structure for coniine.
Conyrine loses two carbon atoms on oxidation which implies that the side chain of coniine consists of three carbon atoms, that is a n-propyl or iso-propyl group. When coniine is heated with HI at 300oC under pressure n-octane is formed rather than iso-octane inferring that the side chain is not branched.
From this evidence it was expected that coniine is 2-n-propylpiperidine and this was confirmed by synthesis in 1886.