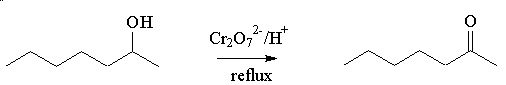| |
"Then there are the English cheeses. There are not many of them but I fancy
that Stilton is the best cheese of its type in the world, with Wensleydale not far behind."
George Orwell, "In Defence of English Cooking",
first published in the Evening Standard on 15 December 1945. |
|
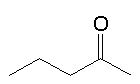
My textbook says heptan-2-one is the molecule causing the smell of blue cheeses like Stilton
Your textbook is partly right. The "blue cheese" note is due to two methyl ketones (alkan-2-ones), heptan-2-one and nonan-2-one. Some blue cheeses have significant amounts of pentan-2-one too, but that molecule has a rather different smell.
Pentanone
|

Heptanone
|

Nonanone
|
Why those?
The smell of ketones depends upon the chain size. Heptan-2-one and nonan-2-one are the only two ketones to have a "blue cheese" character to their smell.
| Propanone | Nail varnish remover |
| Butanone | Similar to propanone |
| Pentan-2-one | Malty, fruity |
| Hexan-2-one | Fruity, floral |
| Heptan-2-one | Blue cheese |
| Octan-2-one | Fruity, floral, musty |
| Nonan-2-one | Blue cheese, floral, fruity |
| Decan-2-one | Fruity, musty |
| Undecan-2-one | Musty, fruity |
|
 |
Are there other ketones in the cheese?
The most abundant ketones in Blue cheeses have five, seven or nine carbon atoms. Heptan-2-one is the most abundant ketone in Blue Stilton, with significant amounts of butan-2-one and pentan-2-one. Heptan-2-one and nonan-2-one are the most abundant in Roquefort and Bleu d'Auvergne, but pentan-2-one is the most abundant in Bleu des Causses, whilst in the Italian blue cheese, Gorgonzola, nonan-2-one is most abundant, followed by heptan-2-one and then undecan-2-one. Pentan-2-one, heptan-2-one and nonan-2-one are all found in Spanish Cabrales Blue cheese, with 2-pentanone and heptan-2-one the most abundant whilst in Gamonedo Blue, it was heptan-2-one and nonan-2-one that were most common.

Roquefort
|

Gorgonzola
|

Cashel
|

Shropshire Blue
|

Bleu d'Auvergne
|

Cabrales Blue
|
Why is it odd-numbered ketones?
Like other blue cheeses, Stilton has Penicillum roqueforti culture added to the milk before rennin is put in to make the curds form - these are separated and made into the cheese. After the cheeses have ripened for a few weeks, they are pierced with stainless-steel needles, allowing air in and speeding up the ripening process, when the characteristic blue veins form.
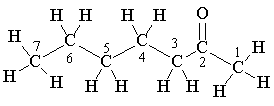
The ketones are derived from the carboxylic acids present in triglycerides in fats; these acids generally contain an even number of carbon atoms. The triglycerides are hydrolysed by the starter bacteria in the cheese forming the free acids, which are then catabolised by the Penicillum roqueforti, being successively oxidized to β-hydroxyacids and to β-ketoacids, before decarboxylation to ketones which have one carbon atom fewer than the initial acid. Thus the even-carbon acids generally found in lipids form odd-carbon ketones, and heptan-2-one is formed from octanoic acid. However, there are only small amounts of short-chain C5 to C10 acids in lipids. It may be that repeated β-oxidation of larger molecules like octadecanoic acid, C17H35COOH, generates much of these small alkan-2-ones.
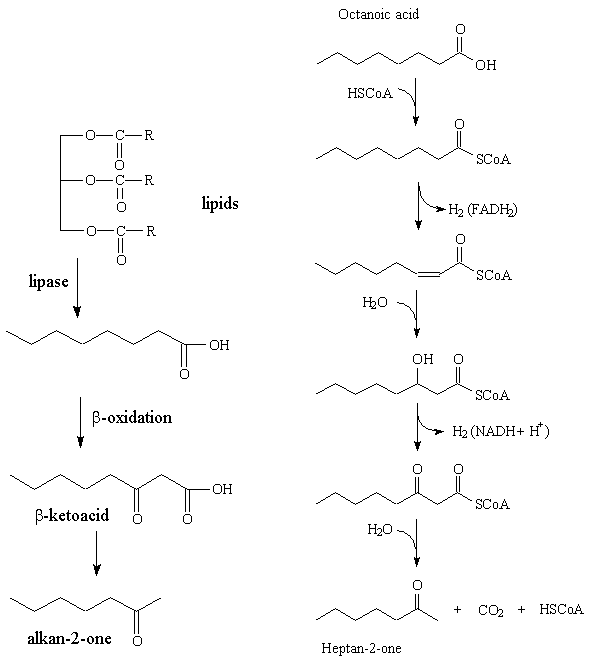
Left: The "big picture" in generating an alkan-2-one, starting from a lipid.
Right: Detailed steps in the formation of heptan-2-one from octanoic acid.
Why do the amounts of the different ketones vary?
There are probably a number of factors involved. For one thing, the composition of the lipids in the milk will vary from one animal to another (cow, sheep, goat).
Where else does heptan-2-one crop up in nature?
Heptan-2-one occur in a few insects. It has long been believed to be an alarm pheromone for bees, but is now thought to be a forage marker. It is a component of the alarm pheromone for some ants, including Iridomyrmex pruinosus and the Texas leafcutting ant, Atta texana, and is believed to be a sex pheromone of the Swedish caddis flies, Rhyacophila nubile and R. fasciata. Along with octan-2-one and nonan-2-one, heptan-2-one is a component of the defensive secretion of Chinese whip scorpions of the order Typopeltis.

Texan leaf-cutter ant
|

Caddis Fly
|
And what is it like?
Heptan-2-one is a colourless liquid at room temperature. Its melting point is -35.5°C and its boiling point is 151°C.
How do you make it in the lab?
It is made by oxidation of the secondary alcohol, heptan-2-ol. This can be done by heating heptan-2-ol with oxidising agents such as acidified potassium dichromate or permanganate, and distilling off the ketone.
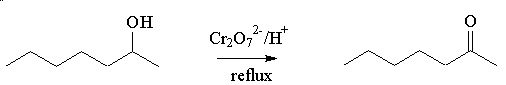
How do you know that it is heptan-2-one?
It could, of course, be one of the other heptanones, heptan-3-one or heptan-4-one. A classic test to tell them apart is that only heptan-2-one gives a positive triiodomethane (iodoform; CHI3) test, being a methyl ketone. It reacts (within minutes) with alkaline iodine solution to form a yellow precipitate of triiodomethane.
RCOCH3 + 3 I2 + 4 NaOH 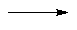 RCO2Na + 3 NaI + 3 H2O + CHI3
RCO2Na + 3 NaI + 3 H2O + CHI3
The reaction happens because on replacement of the methyl hydrogens by iodine, the CI3 group is sufficiently electron withdrawing to weaken the C-CI3 bond, so that it is readily replaced by OH-.
RCOCH3 + 3 I2 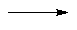 RCOCI3 + 3 HI
RCOCI3 + 3 HI
This test is given by most compounds with a CH3C=O grouping, meaning methyl ketones and ethanal; also compounds that can be oxidized to the CH3C=O group, such as heptan-2-ol.
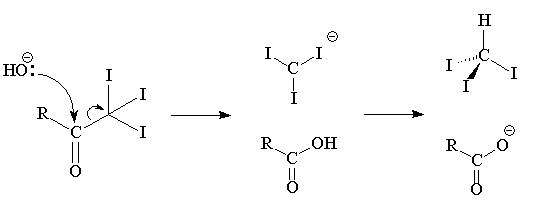
|

Positive and Negative iodoform test
|
Can't you identify it using spectroscopy?
Indeed. The C=O stretching frequency in the infrared spectrum is at ~1718 cm-1, showing that it is a carbonyl compound. The Mass Spectrum shows the molecular ion with m/z = 114 (and a carbon-13 satellite at 115); the prominent fragment occurs at m/z = 43, due to C3H7+ and to CH3CO+. The 1H-NMR spectrum is a key to its identification, showing six signals, one for each set of hydrogens; the signal due to the hydrogens bound to carbon-1 is unsplit, as there are no neighbouring hydrogens, but all the other signals show spin-spin splitting.
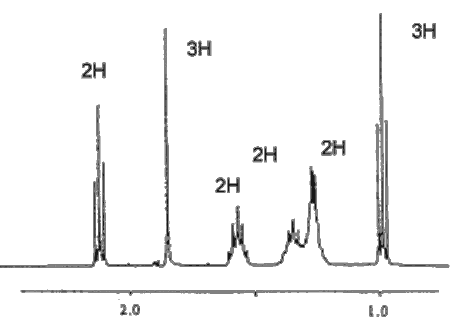 |
| (ppm) | Intensity | Description | Carbon to which
H's are bound |
| 0.97 | 3 | Triplet | 7 |
| 1.3 | 2 | Multiplet | 6 |
| 1.4 | 2 | Multiplet | 5 |
| 1.6 | 2 | Multiplet | 4 |
| 1.8 | 3 | Singlet | 1 |
| 2.2 | 2 | Triplet | 3 |
 |

Bibliography
General
- Chapman and Hall Combined Chemical Dictionary, compound code number DBM14-A (props, bibliography)
- S. Sablé and G. Cottenceau, J. Agric. Food. Chem., (1999) 47, 4825 (cheese odorants)
Ketones in blue cheeses
- J. B. Lawlor, C. M. Delahunty, J. Sheehan and M. G. Wilkinson, Int. Dairy J., (2003) 13, 481. (blue cheeses)
- K. Gkatzionis, R. S. T. Linforth and C. E. R. Dodd, Food Chemistry, (2009) 113, 506. (Stilton)
- A. Gallois and D. Langlois, Lait, (1990) 70, 89. (Roquefort and other French blue cheeses).
- L. Moio, P. Piombino and F. Addeo, J. Dairy Res., (2000) 67, 273. (Gorgonzola)
- A. B. Flórez, P. Ruas-Madiedo, L. Alonso and B. Mayo, Eur. Food Res. Technol., (2006) 222, 250. (Cabrales)
- D. Gonzalez de Llano, M. Ramos, C. Polo, J. Saw and I. Martinez-Castro, J. Dairy Sci., (1990) 73, 1676. (Gamonedo)
Heptan-2-one in insects
- J. B. Free, Pheromones of social bees, Springer, 1987.
- M. S. Blum, S. L. Warter, R. S. Monroe and J. C. Chidester, J. Insect Physiol., (1963) 9, 881 (Iridomyrmex pruinosus)
- M. S. Blum, J. G. Traynham and S. L. Warter, J. Insect Physiol., (1966) 12, 419. (Iridomyrmex pruinosus)
- A. Vallet, P. Cassier and Y. Lensky, J. Insect Physiol., (1991) 37, 789. (bees)
- J. C. Moser, R. C. Brownlee and R. M. Silverstein, J. Insect Physiol., (1968) 14, 529. (Atta texana)
- R. G. Riley, R. M. Silverstein and J. C. Moser, J. Insect Physiol., (1974) 20, 1629. (Atta texana)
- C. Löfstedt, B. S. Hansson, E. Petersson, P. Valeur and A. Richards, J. Chem. Ecol., (1994) 20, 153. (caddis fly)
- M. C. Larsson and B. S. Hansson, J. Insect Physiol., (1998) 44, 189. (caddis fly)
- J. Haupt, G. Höhne, H. Schwarz, B.- S. Chen, W.-B. Zhao, and Y.-C. Zhang, J. Comp. Physiol. B, (1988) 157, 883. (Chinese scorpion)
Spectra
Triiodomethane test
- F. G. Mann and B. C. Saunders, Practical Organic Chemistry, Longman, London, 4th edition, 1960, pp 334, 344, 350.
- B. S. Furniss et al, Vogel's Textbook of Practical Organic Chemistry, Longman, London, 1989, p. 1220.
- http://www.chemguide.co.uk/organicprops/carbonyls/iodoform.html
- http://www.youtube.com/watch?v=DMWfSdlZuF8
- http://www.creative-chemistry.org.uk/alevel/module3/documents/N-ch3-16.pdf
- http://www.wellesley.edu/Chemistry/chem211lab/Orgo_Lab_Manual/Appendix/ClassificationTests/aldehyde_ketone.html#Iodoform
Acknowledgement
The NMR spectrum of heptan-2-one is reproduced by kind permission of Professor Les Field of the University of Sydney.


 Back to Molecule of the Month page.
[DOI:10.6084/m9.figshare.5255173]
Back to Molecule of the Month page.
[DOI:10.6084/m9.figshare.5255173]

![]()
![]()
![]()
![]()