
Ibuprofen
 |
By John Bower, e-mail: jb9663@bris.ac.uk
School of Chemistry, University of Bristol
Molecule of the Month - November 2001
Introduction
Ibuprofen molecule
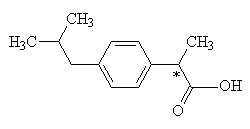 |
Molecular Information
IUPAC name= 2-(4-Isobutylphenyl)propanoic acid
Molecular Weight = 206.29 g mol-1Molecular Formula = C13H18O2
Molecular Composition = 75.69 % C, 8.8 % H, 15.51 % O
Melting Point = 75 - 77 oC
Number of Chiral Centers (stereocentres) = 1
Ibuprofen, a carboxylic acid, was first introduced in the UK 1969 by the Boot Pure Drug company under the trade name Brufen. It belongs to a class of drugs known as non-steroidal anti-inflammatory drugs or NSAIDs of which there are now more than 50 on the market. Ibuprofen has a powdery white appearance and can come in the form of capsules, tablets, or powder. Today it is sold under several trade names such as Advil, Motrin and Nuprin:-

It is often prescribed for "rheumatic" musculoskeletal complaints and is taken without prescription for minor aches and pains. Ibuprofen is the first choice drug in its class since it has a low incidence of unwanted effects. It is rapidly metabolized, usually leaving the body via urination within 24 hours.
Because ibuprofen has only one stereocentre, it can exist in two enantiomeric forms. The commercially available product is usually the racemate since it is difficult to separate the two enantiomers.The biologically active form is the S enantiomer and the R form is converted to this within the body thus minimizing side effects (c.f. thalidomide problem of the 1950's and 1960's). One may have assumed, therefore, that only 50% of the drug would be active but this is not the case due to biologically catalysed enantiomeric interconversion.
Synthesis
Two synthetic routes to ibuprofen:-
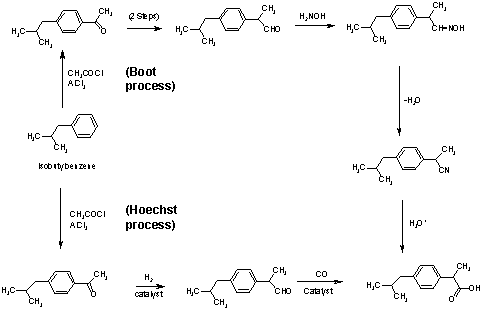
(This image was copied from www.chem.vt.edu without permission)
There have been many commercial and laboratory publications for the synthesis of Ibuprofen. Two of the most popular ways to obtain Ibuprofen are the Boot process and the Hoechst process. The Boot process is an older commercial process developed by the Boot Pure Drug Company, and the Hoechst process is a newer process developed by the Hoechst Company. Most of these routes to Ibuprofen begin with isobutylbenzene and use Friedel-Crafts acylation. The Boot process requires six steps, while the Hoechst process, with the assistance of catalysts, is completed in only three steps.
Cheminor Drugs have developed a process for an improved version of ibuprofen based on chiral synthesis. The move is significant given that pure S-Ibuprofen (the active form of ibuprofen) could near halve the regular ibuprofen dosage, besides improving the side-effect profile.
However the human body can convert the inactive (R) form into the (S) form, so eventually 100% of the ibuprofen taken becomes active. The process discovered by Cheminor is therefore unlikely to have commercial significance.
Metabolism
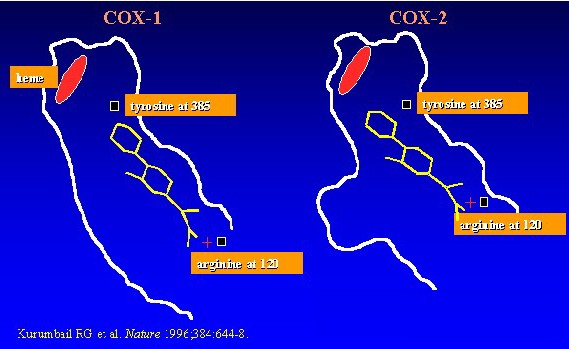
Ibuprofen is a non-steroidal anti-inflammatory agent belonging to a group of propionic acid derivatives. It has three major types of effect which are all linked to its primary action, the inhibition of an enzyme known as arachidonate cyclooxygenase or COX of which there are two types COX-1 and COX-2
COX-1 and COX-2 inhibition by NSAIDs
The cyclooxygenase pathway:-
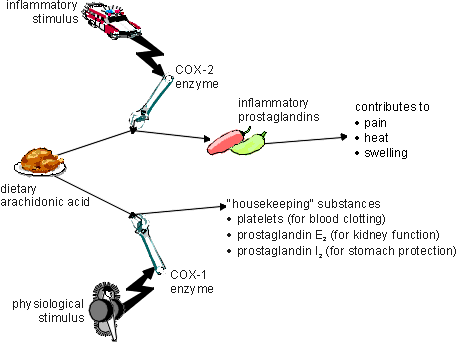
(This image was copied from http://elfstrom.com without permission)
The inhibition of COX-2 inhibits the production of prostaglandins and thromboxanes. This, in turn, leads to the following three major effects:-
Anti-inflammatory effect: modification of inflammatory reactions via decrease in vasodilator prostaglandins.
Analgesic effect: reduction of certain sorts of pain via reduced sensitivity of nerves to certain inflammatory mediators.
Antipyretic effect: lowering of a raised temperature via decrease in a mediator prostaglandin which is responsible for elevating the hypothalamic temperature control.
Unwanted effects are mainly due to the inhibition of COX-1 since this is only concerned with the production of housekeeping substances and not related to prostaglandin synthesis.
Uses and Side Effects
Uses
Ibuprofen is used to relief the symptoms of a wide range of illnesses such as headaches, backache, period pain, dental pain, neuralgia, rheumatic pain, muscular pain, migraine, cold and flu symptoms and arthritis.
Recently evidence has emerged suggesting that ibuprofen is effective in the treatment of Alzheimer's disease (move mouse over picture):-
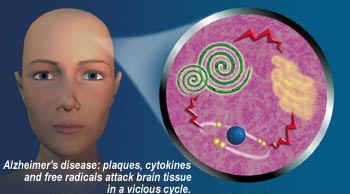
(Image taken without permission)
Side Effects
Ibuprofen is regarded as the first choice drug in its class due to the low number of side effects and complications associated with it.
The most frequent type of adverse reaction occurring with ibuprofen is gastrointestinal. In clinical trials, the percentage of patients reporting one or more gastrointestinal complaints ranged from 4% to 16%.
In studies when ibuprofen was compared to aspirin and indomethacin in equally effective doses, the overall incidence of gastrointestinal complaints was about half that seen in either the aspirin or indomethacin treated patients.
| Common Side Effects | stomach upset or irritation |
| Infrequent Side Effects | nausea and/or vomiting, constipation, diarrhea |
| Rare Side Effects | skin irritations, drowsiness, gastrointestinal bleeding |
| Risks | liver toxicity, stomach ulcers, allergic reactions |
The adverse effects of ibuprofen and other NSAIDs
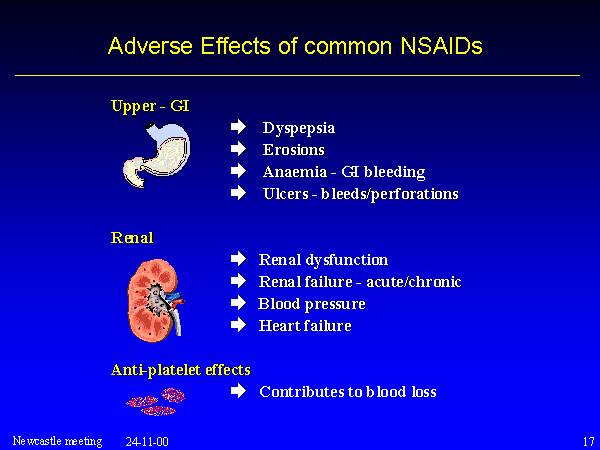
References
http://elfstrom.com/arthritis/nsaids/actions.html
Rang H.P., Dale M.M., Ritter J.M., Pharmacology, 4th edition, London, 1999.
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5462158]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5462158]