
![]()
INCENSOLE
and other molecules in frankincense,
including incensole acetate.
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month December 2017
Also available: JSMol version.
![]()
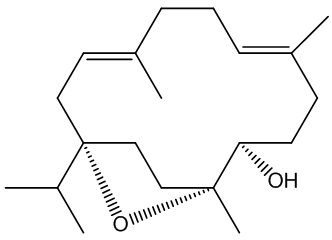
Incensole
 |
INCENSOLEand other molecules in frankincense,
|
 Incensole |
 By 'Holy Smoke', I assume you mean the incense used in church services?
By 'Holy Smoke', I assume you mean the incense used in church services?Yes.
One of them, yes. The resin frankincense (famous as one of the gifts the 3 Wise Men gave to the baby Jesus in the Bible story), is often used in many religious ceremonies and burned to give a sweet-smelling odour. Incense was used by the Ancient Egyptians, the Chinese, and the early Christians, and is still a vital part of many religious ceremonies, including Buddhist, Taoist, Shinto and of course Christian. The smoke of burning incense is interpreted by both the Western Catholic and Eastern Orthodox Christian churches as a symbol of the prayer of the faithful rising to heaven. However, a more practical reason for its early use in medieval churches was probably to mask the smell of the unwashed congregation!
The most well-known are the boswellic acids, but in fact over 300 volatile molecules have been identified in frankincense, including incensole.
Money does not grow on trees, but frankincense does. It is the solidified resin that emanates from trees of the Boswellia spp., which mainly grows in East Africa and India. These species include B. papyrifera associated with Ethiopia; B. sacra with East Africa and B. serrata with India. According to the great Greek chronicler Herodotus (ca. 484–425 BC), flying snakes known as drakontes lived under frankincense trees to guard them, but the snakes could be driven away by burning resin of the Liquidambar tree.
 |
 |
| Boswellia papyrifera tree | Harvesting frankinsence |
Extraction of cold powdered frankincense with petroleum ether gives a yellow-coloured oil. Filtration over neutral alumina removes acidic constituents and further work-up, including treatment with the reducing agent LiAlH4 enables incensole to be isolated in nearly 4% yield. An alternative route utilises extraction with diethyl ether.
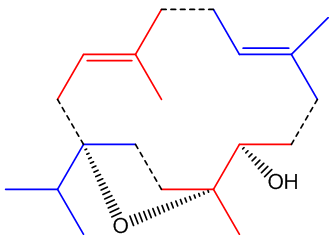 Its structure looks a bit odd.
Its structure looks a bit odd.Incensole is a diterpenoid, one of many chemicals in nature which are structurally derived from isoprene, 2-methyl-1,3-butadiene (MOTM July 2008).
The diagram (right) shows how the four isoprene units are joined in the structure, though its biosynthesis will be much more complicated. In other words, though it contains isoprene units, it is not made from isoprene.
In particular it is known as a cembranoid; these are diterpenoids found in many natural places, plants like tobacco and some conifers and frankincense, plus marine organisms (including coral and sponges), marine invertebrates and insects. It also has three asymmetric (chiral) carbon atoms, denoted by * in this diagram.
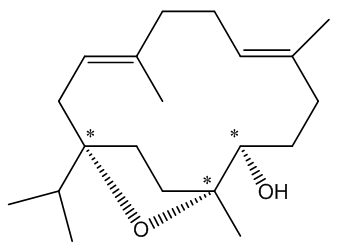
The chiral centres in incensole.
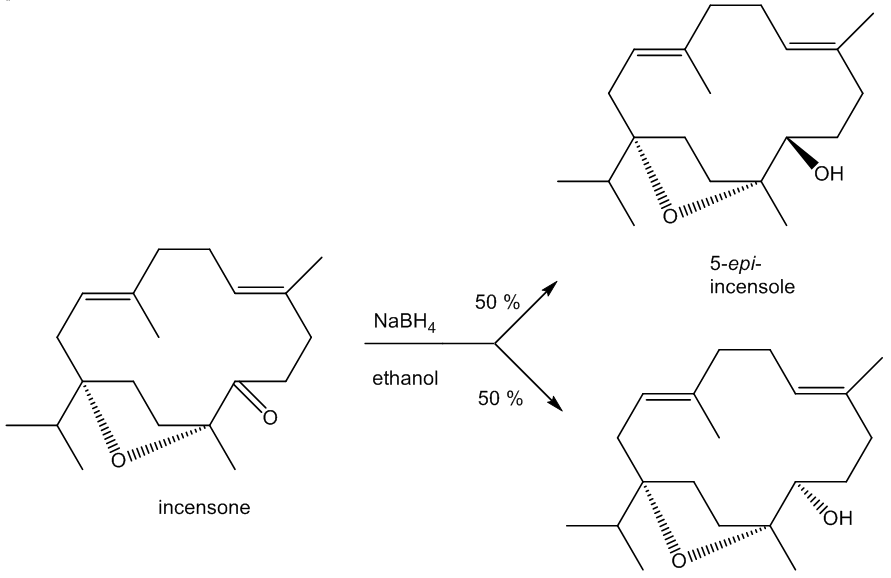
Incensole is a secondary alcohol, so it can be acetylated using acetic anhydride to produce the ester incensole acetate (other esters can similarly be made) and oxidised to a ketone, known as incensone.
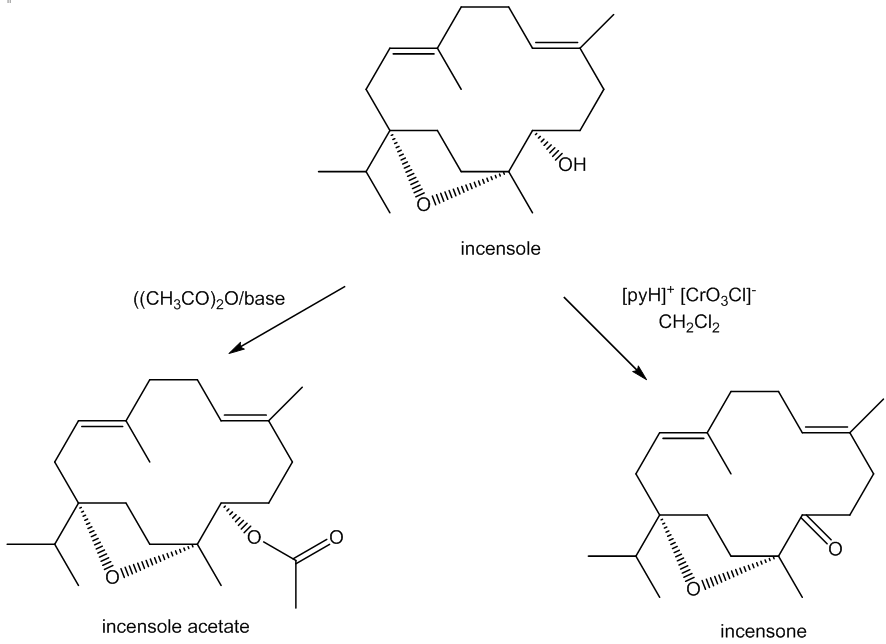
The C=O group in incensone can be reduced back to an –OH group. Because the reducing agent can approach the carbonyl group either from ‘above’ or ‘below’, two isomeric products are obtained in equal amounts, incensole and its ‘epimer’ 5-epi-incensole. (An epimer only differs from its isomer at one asymmetric carbon). Nature only makes the single isomer as the enzymes involved in the biosynthesis of incensole have restricted active sites and so they exert tighter control over the molecule’s stereochemistry.
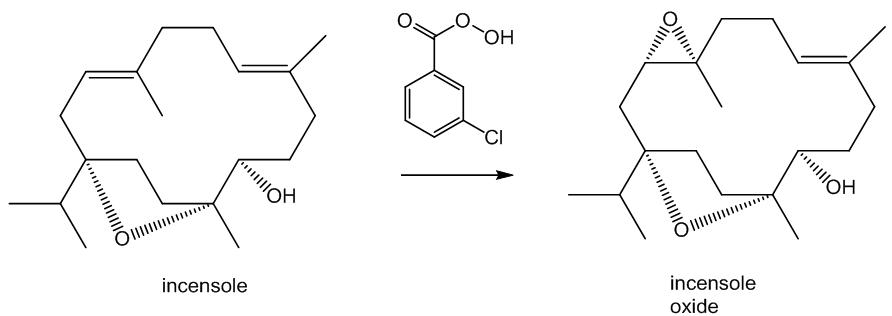
It can also be oxidised in a slightly different way. Reaction with meta-chloroperoxybenzoic acid (mCPBA) affords incensole oxide by epoxidation (the Prilzhaev reaction). This reaction also occurs on keeping incensole in contact with air.
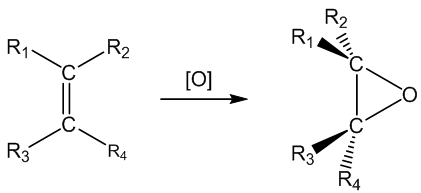
It means using oxidation to create an epoxide; this often involves oxidation of a C=C bond. Epoxides are cyclic ethers which contain a three-membered ring. Because of the bond angles involved, these rings are strained, so epoxides are usually quite reactive.
Apart from its use in worship, frankincense has been used medicinally in the East for thousands of years, but recently scientists in the West have started to examine it, and find that incensole acetate in particular has some interesting properties.
In 2008 it was discovered that the smoke from burning frankincense relieved depression and anxiety in mice; incensole acetate was identified as the compound responsible for this. Incensole acetate interacts with a receptor known as TRPV3, short for Transient Receptor Potential cation channel, sub-family V, member 3 (the related TRPV1 receptor is the one that the chilli pepper molecule capsaicin (MOTM April 2001) interacts with, causing curries to seem ‘hot’). The TRPV3 receptor is found in the skin, where activating it gives a warm sensation, and is also found in the brain. Incensole acetate activates the amygdala and septum, as well as the motor cortex. Incensole acetate has also been linked with neuroprotective and anti-inflammatory effects, so scientists wonder if it may be useful in treating brain injuries.
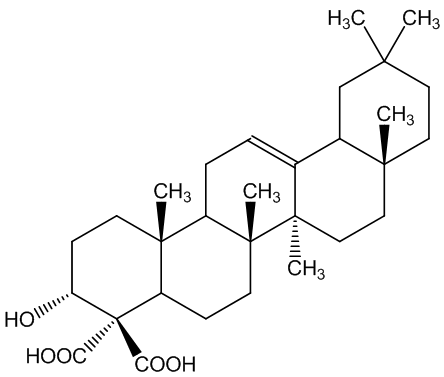
The boswellic acids (MOTM February 2000) continue to be of interest. Archaeologists use their detection using their characteristic mass spectra (MS) to find frankincense in ancient artefacts.
 |
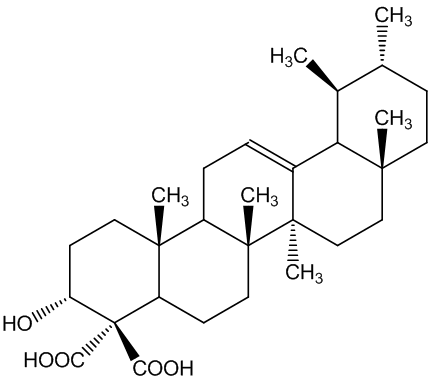 |
| α-boswellic acid | β-boswellic acid |
They are also believed to have anti-inflammatory properties that could make them useful in treating complaints like rheumatoid arthritis. Most recently it has been suggested they may have a role in cancer therapy.
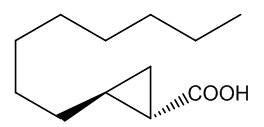
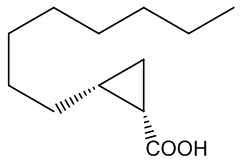
No, that is down to another type of molecule. In 2016 a group of researchers in France and Italy reported that they had found two very similar molecules that were responsible for the characteristic ‘endnote’ of frankincense, an ‘old church’ smell. They are (+)-trans- and (+)-cis-2-octylcyclopropyl-1-carboxylic acids.
 |
 |
 |
| (+)-trans-2-octylcyclopropyl-1-carboxylic acid | (+)-cis-2-octylcyclopropyl-1-carboxylic acid | Incense leaves behind the 'old church' smell |
These molecules have very intense smells, but are only present in tiny amounts in frankincense. The researchers distilled 3 kg of frankincense (under reduced pressure, because so many of the molecules are very large and have high boiling points). The key odorant was present in a fraction that made up only 0.2% of the oil. The researchers carried out further separations on this fraction and obtained a 1 mg sample that contained the molecules responsible, less than one-in-a-millionth of the original sample. They then did spectroscopic measurements on them, and worked out what their structures were. These molecules had never been made before, so the scientists devised a way of making them ‘from scratch’ to confirm their identities.
Yes, one unusual feature of the structures is the presence of the three-membered cyclopropyl rings, which are very uncommon, because of the ring-strain (the internal ring bond angles ~ 60°, compared with the normal 109½° angle for saturated (sp3 hybridised) carbon. Although the molecules lack the C=C bond usually needed for such isomerism, the ring system to which they are attached prevents free rotation between the two isomeric forms in the same way that the π-bond does in alkenes. Special smell, special molecules.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5661844]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5661844]