
Indigotin: the dye used to colour bluejeans.
|

Indigotin
The dye used to colour blue jeans.

Richard Marsh and Paul May
Bristol University, UK

Molecule of the Month February 2009
Also available: HTML version.

|

A Toureg tribesman with an indigo-dyed headscarf.
|
People are dyeing to use it
Indigo is an ancient compound, and has been known and used as a distinctive blue dye since prehistoric times [1]. The earliest users (and exporters to Europe) were from India, and the country gained its English name from the ancient Greek word for indigo [9]. Historically, indigo played an important role in the economies of many countries because natural blue dyes are rare. Today it will be most familiar to you as the dye used to colour blue jeans.

Yarn dyed with Indigo
Indigo in its synthesised or purified form is insoluble in water and other polar solvents. To overcome this problem, the dye is reduced to soluble leucoindigo (known as 'white indigo'), and applied to clothes in this form. When exposed to atmospheric oxygen it re-oxidises to the insoluble form and regains its colour. Originally this reduction was done with urine, although synthetic urea replaced it in the 19th century and later sodium hydrosulfite was employed as a much more effective reducing agent [7]. Once the problem of applying the dye to the clothing had been overcome the insolubility of the dye is of course beneficial - it will not wash out of the fabric in water.


Indigo powder plant extract Lump of indigo dye from India
Although indigo has been primarily used in recent centuries for dyeing cotton (often for denim jeans and jackets), it can also be applied to polyesters by the same method. When indigo is reduced by the sodium hydrosulfite in sodium hydroxide, three possible reduction products can result, mono- and di-ionic products resulting from the carbonyl oxygen forming an ionic bond with the sodium cations, or a non-ionic form where the oxygen bonds to a proton. By manipulation of the hydrosulphite and hydroxide concentrations the reaction can be tailored to produce the reduction product best suited to the fabric being dyed [3].
 I got the blues...
I got the blues...
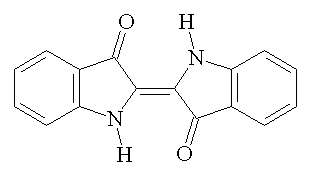
The chemical in indigo which is responsible for the blue colour is indigotin (structure below), which is a dark blue powder at room temperature (see photo, above) and is insoluble in water and ethanol [2]. It is most soluble in chloroform, nitrobenzene and sulphuric acid [3]. The structure was first published in 1883 by Adolf von Baeyer (photo, right) who won the Nobel Prize for his work on the dye in 1905, along with theories of what would become known as Baeyer strain in cyclic compounds. (It is no coincidence that the dye he worked so extensively on contains both five and six membered rings. [4])
 |
 |
|
| Indigotin |
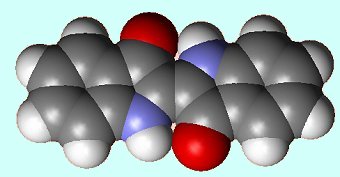
...and its spacefill structure. |
|
Production and Synthesis
Indigo has been known since ancient times and originally came from a plant extract. Plants of the Indigofera genus contain a glycoside called indican in their leaves and stems, which is extracted, and acid-hydrolyzed into indoxyl [5]. Mild oxidation in atmospheric oxygen will then produce indigo [6].
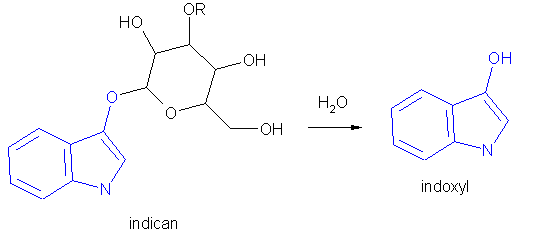
There are obvious disadvantages to relying on plants for the indican necessary to make the indigo dye - it is used in vast quantities in the fabric industry today. Luckily, soon after his discovery of its structure, Baeyer developed a synthesis route for the product, using acetic acid and sodium hydroxide to produce an indoxyl isomer in quantity [7]. Soon after Baeyer, the Heumann process was also developed, which used sodium hydroxide and phenylglycine-o-carboxylic acid under high temperature and pressure to produce indoxyl [8].
Finding the Dye - Analysis
There is significant incentive in the art world to develop techniques which can determine what chemicals are present in the paint on old masterpieces, for the purposes of historical research, to ensure accuracy during restoration, to determine the best methods of conservation and to identify forgeries and copies. Obviously it is not desirable to remove large amounts of paint so techniques are developed which provide accurate analysis of the tiniest samples.
 One such method involves the use of reversed-phase high performance liquid chromatography (HPLC), in which the sample is pumped at high pressure through a stationary phase to determine component polarity based on retention times, coupled to an electrospray ionisation mass spectrometer to determine the molecular mass of the species as they arrive at the end of the chromatography column [10]. This technique was used by Puchalska et al. [21] to detect indigotin in concentrations as low as 0.03 μg ml-1, and can be used to detect not only the presence of indigo but to determine the origin of the dye with some accuracy as different production and dyeing methods throughout history led to different exact chemical compositions, impurities and affinity to the canvas.
One such method involves the use of reversed-phase high performance liquid chromatography (HPLC), in which the sample is pumped at high pressure through a stationary phase to determine component polarity based on retention times, coupled to an electrospray ionisation mass spectrometer to determine the molecular mass of the species as they arrive at the end of the chromatography column [10]. This technique was used by Puchalska et al. [21] to detect indigotin in concentrations as low as 0.03 μg ml-1, and can be used to detect not only the presence of indigo but to determine the origin of the dye with some accuracy as different production and dyeing methods throughout history led to different exact chemical compositions, impurities and affinity to the canvas.
Ortega-AvilÚs et al. [12] detail a variety of other techniques which can be used to determine the composition of a painting, in their case a work titled 'Virgin of Sorrows' (photo, right) which purported to be an 'ancient' piece. Raman and infra-red spectra were taken of the microsamples taken from the painting and the results analysed to determine both the compounds present and specific signatures which betray the origin of the dyes. Specific impurities and methods of binding to canvas fibres help to determine, using data from known samples, where the dyes used came from, when they were made, and which methods were used to extract or synthesise them. The authors found that the painting was elaborated during the middle of the 18th Century or the early third of the 19th Century.

References
Many images on this page were taken from Wikimedia Commons.
- Wikipedia - indigotin
- NIST Chemistry WebBook
- Kunitou K,, Textile Res. J., 75, (2005) 149.
- Encylcopedia Britannica Online
- Wu E,, et al, Biotech. Techn., 13, (1999) 567.
- Clayden et. al, Organic Chemistry, (Oxford University Press, New York, 2001 p.171)
- McKee J,R,, J. Chem. Ed., 68, (1991) 10.
- Hunger K, et al, in Industrial Dyes, ed. K Hunger, (Wiley, 2004, 13).
- Mattson A,, Indigo in the Early Modern World
- Dorsey J,G., et al, Anal. Chem., 64, (1992) 353.
- Puchalska M., et al, J. Mass Spectrom., 39 (2004) 1441.
- Ortega-AvilÚs M., et al, Anal. Chimica Acta, 550, (2005) 164.


 Back to Molecule of the Month page.
Back to Molecule of the Month page.

![]()
![]()
![]()
![]()




 I got the blues...
I got the blues...


