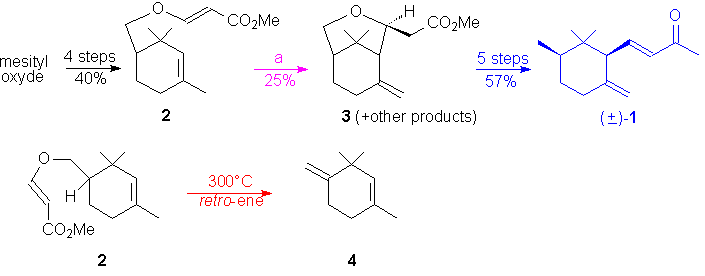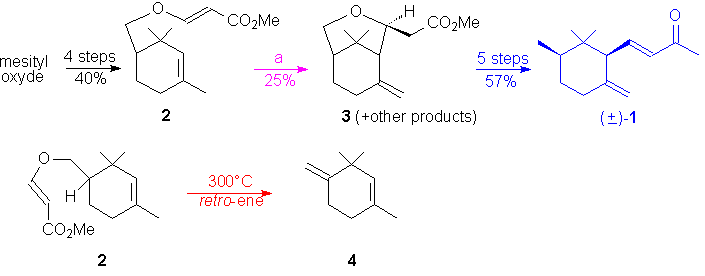Silicon-Controlled Intramolecular
Thermal H-ENE Reaction.
A New Access to a Key Intermediate
of (±)-Cis-g-Irone.
Honoré Monti,* Gilles Laval, and Michel
Féraud
-
The synthesis of Fráter suffers in the
cyclization's step from a modest 25 % yield of the expected bicyclic compound
3
and
greatly penalyzes, at the crucial stage, the overall yield of the synthesis.
The major drawback is the formation of a side product, 1,3,3-trimethyl-4-methylidene-1-cyclohexene
4
(ca. 40%, see below), by a competitive retro-ene reaction.
As part of our interest
in the ene reaction of allylsilanes [26],
[27],
we report a significant improvement in the diastereoselective synthesis
of new access to a the key intermediate 3 starting from Hagemann's
ester 5 in the synthesis of (±)-cis-g-irone
with high chemocontrol thermal H-ene reaction and significant improved
overall yield.
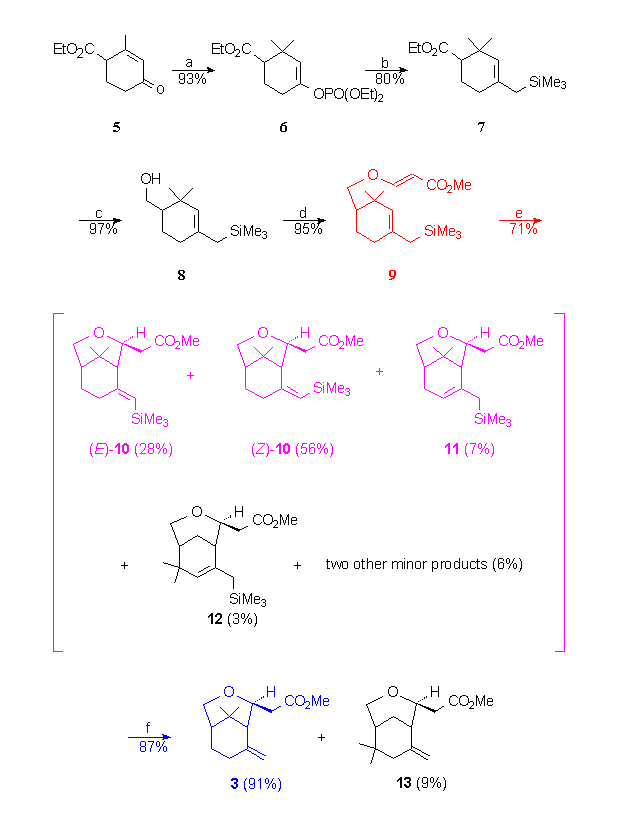
-
Reagents : a) i. 2 MeLi, CuI, Et2O,
-5°C ii. HMPT, ClPO(OEt)2, THF; b) ClMgCH2SiMe3,
NiCl2(dppp) cat., THF, reflux; c) LiAlH4, Et2O,
-20°C then rt.; d) methyl propiolate, N-methylmorpholine, Et2O,
rt.; e) Decaline, sealed tube, 250°C; f) p-toluene sulfinic
acid , CH2Cl2, rt.
-
In summary, the intramolecular
thermal H-ene reaction of an allysilane has been studied in terms of chemo-
and regioselectivity. This has been developed into a strategy for a new
access to a key intermediate in the diastereoselective synthesis of(±)-cis-g-irone
with
total chemocontrol and 56% overall yield.
-
If you desired to go into that more deeply, read
the publication.
-