This chapter is based on "Scent and Fragrances, Günther Ohloff " & "Common Fragrance and Flavour Materials, Kurt Bauer, Dorothea Garbe, Horst Surburg ".
- Similarity between odors arises because similar
substances or mixtures of compounds may interact
with receptors to create similar
sensory impressions in the sensory centers of the brain. The group
of musk fragrances (comprising macrocyclic ketones and esters as well as
aromatic nitro compounds and isochroman derivatives) are, for example,
compounds with similar odors but totally different
structures.
Small changes in structure (e.g., the introduction of one or more double bonds in aliphatic alcohols or aldehydes) may, however, after a sensory impression or intensify an odor by severals orders of magnitude. Increasing knowledge of the structure and functioning of olfactory receptors may provide a better scientic basis for the correlation of odor and structure in fragrance and flavour substances, and facilitate the more accurate prediction of the odor of still unknown compounds.
1. Regioselectivity of Odor Perception
Using
an appropriate model, the dependance of the odor properties of an organic
compound on structural modifications can be readily demonstrated. In the
simplest case one can affect an odor change on an odorant by changing
the molecular environment of the functional group.
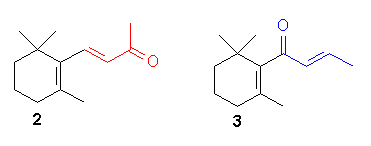
A simple transposition of
the allyl function in an odorant can lead to drastic
changes in odor quality. A good example in
b-ionone
2
the odor of which after transposition into "roseketone" 3 changed
from the typical violet character to a complex odor which very fruity,
camphoraceous and green tonalities.
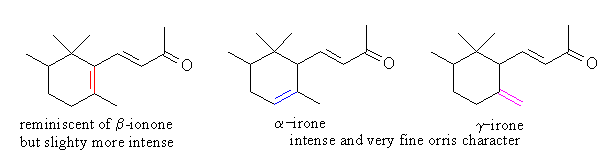
a-, g- double bond isomers of irone have iris-like qualitatively similar odor, but the odor of b-irone is quite similar of b-ionone (reminiscent of cedarwood, violet-like upon dilution).
2. Stereochemistry and Odor
Influence
of Substituents:
Functional groups play a determining part on the strengh and character
of odorants. The driving force of the odor receptor event is considered
to be a directed dipole-dipole interaction.
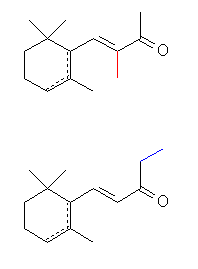 a-isomethylionone
: reminiscent of orris and violets, possesses the finest odor of all ionones.
a-isomethylionone
: reminiscent of orris and violets, possesses the finest odor of all ionones.
b-isomethylionone : interesting, powdery, orris-like with woody aspects.
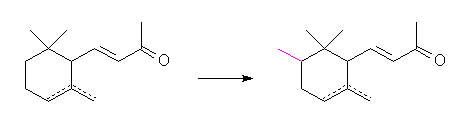
a-, g-
irones, in that a methyl group is in the ring are remarkable substances
possessing a powerful and most pleasant violet odor much superior to any
ionone and methylionone.
Geometry of Unsatured Compounds: The (E, Z) isomeric odorants which generally exhibit different sensory profiles. There is remakable difference in the odor character between the artificial violet odorant (E)-8-methyl-a-ionone 4 and its pleasant, woody, tobacco-like (Z)-isomer 5.
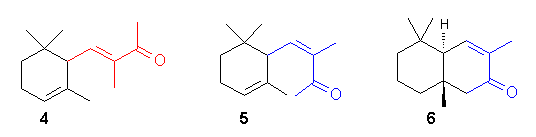
- Apparently the different shapes of the molecule
determine the odor quality, because the bicyclic analog 6 has a
very similar odor to the monocyclic 5.
Diastereoselectivity:Small changes in the molecular srtucture of a stimulus can lead to drastic changes in the qualitative odor properties of an odorant.
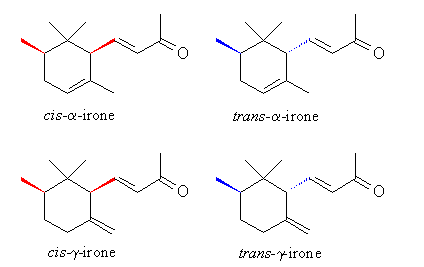 From
the standpoint of aroma quality, cis-a-irone
has a clean iris oil odor, while the diasteromeric trans-a-irone
is less appreciated.
From
the standpoint of aroma quality, cis-a-irone
has a clean iris oil odor, while the diasteromeric trans-a-irone
is less appreciated.
cis-g-irone
possesses a powerful and most pleasant violet odor much superior to trans-g-irone.
3. QSAR (Quantitative-Structure-Activity-Relationships)
Using connectivity parameters as selective descriptors, QSAR studies were done with several types of odorants like the sweaty odor of fatty acids, and compounds that are structurally related to benzaldehyde. The fact that the two of Amoore's "Primary Odors" such as etheral and floral could also be correlated to a corresponding standard compound reduces the value of this correlation procedure. Unfortunately, three dimensional connectivity increments are not known.
Methods that only use physico-chemical
data, as well as topological approaches that use only two dimensions, are
not suited for olfactory studies. A statistical method that is based on
the computer-assisted identification of the sensory active parts of a molecule
for calculating Structure-Activity Relationships (SAR) showed great results.
The value of this pattern recognition analysis depends on the number and
quality of the selected descriptors.
Computer-assisted molecular
modeling handles the molecular properties
of an active compound in three dimensions.
Today, a number of commercially available programs can be used for computer-aided
molecular graphics. The pharmaceuticalndustry also uses tailor-made,
proprietary programs for this purpose.
The
super-imposition of structurally modified
compounds with active molecules not only allows the identification of the
osmophoric structural parts but it can also, to a limited extent, lead
to the prediction of new odorant that possess similar
properties to the compound with which it was
superimposed.
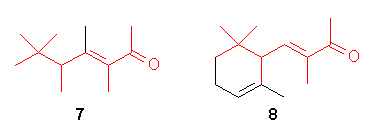
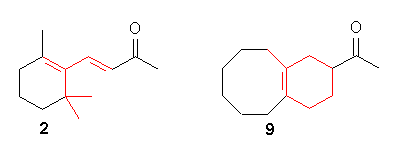
Recently [6], molecular modeling of b-ionone
2
showed that the steric bulk of the dimethyl substitution can be mimic
by fusing a six-membered ring. The remaining 5-methyl group was then found
to be best superimposed by a cyclooctene structure.
Combination of these structural features gave the bicyclic ketone 9.
9 was synthesized and the fruity-woody odor of the tailored
molecular target 9 was indeed found to be very close to that of
the parent b-ionone 2.