|
|
KETAMINE
|
|
|
|
KETAMINE
|
|
Tim Aldridge, University of Bristol, School of Chemistry.
Molecule of the Month - April 2003
Introduction.
|
|
Ketamine is a central nervous system depressant. It is chemically known as (±)-2-(2-Chlorophenyl)-2-(methylamino)cyclohexanone, and belongs to a class of drugs called "dissociative anaesthetics", so called as they separate perception from sensation. Other drugs in this class include PCP and Laughing gas (nitrous oxide). Ketamine was first synthesized in 1962 by a doctor searching for an alternative to the anaesthetic PCP. It was mass-produced by the pharmaceutical company Parke-Davis and was extensively used in the Vietnam War. |
Ketamine was used for anaesthesia because it was seen to suppress breathing much less than most other available anaesthetics, but in the 1970's patients began to report unwanted visions while under its influence.
Today, ketamine is sold commercially in the UK as Ketalar and is widely used as a veterinary anaesthetic. It is mainly used in the treatment of farm animals, although does still have some medical applications in humans.

[Photo taken without permission from www.erowid.org.]
Ketamine is usually found in liquid form, as shown above. It can also be found in powder and often pill form. The similarity in appearance means that ketamine is often mistaken for cocaine.
MOLECULAR INFORMATION - KETAMINE.
| Name | (±)-2-(2-Chlorophenyl)-2-(methylamino)cyclohexanone. |
| Molecular weight | 237.73 g mol-1 |
| Molecular Formula | C13H16ClNO |
| Melting Point | 262-263° C |
| LD50 | 224±4 mg/kg (adult mice) |
Uses and Effects.
Ketamine was reportedly used first as a recreational drug in 1965. Today, it's use as a psychedelic 'Dance' drug is becoming more and more widespread. The recreational use of ketamine was thought to have started in rural areas where access to the drug is relatively easy.
Also known as K, Special K, Vitamin K, Kit Kat, Keller, Super Acid, and Super C, Ketamine is available in tablet, powder, and liquid form. So powerful is the drug that, when injected, there is a risk of losing motor control before the injection is completed. In powder form, the drug can be snorted or sprinkled on tobacco or marijuana and smoked. The effects of Ketamine last from 1 to 6 hours, and it is usually 24–48 hours before the user feels completely “normal” again.

[Photo taken from www.erowid.org without permission]
At lower doses ketamine has a mild, dreamy feeling similar to nitrous oxide. Users report feeling floaty and slightly outside their body. Numbness in the extremities is also common.
Higher doses produce a hallucinogenic effect, and may cause the user to feel very far away from their body. This experience is often referred to as entering a "K-hole" and has been compared to a near death experience with sensations of rising above one's body.
Many users find the experience spiritually significant , while others find it frightening. While in a K-hole it is very difficult to move. People usually remain seated or lying down during the experience.
Dangers and side effects.
|
|
Street ketamine usually originates from legitimate pharmaceutical sources that have been illegally diverted onto the black market. This means that the problems of purity and chemical content, usually associated with street drugs, are absent. However, this does not mean that ketamine is a 'safe' drug. |
The dissociative actions of ketamine mean that it is often considered to be a 'date rape drug'. A date rape drug is one that is slipped into a persons drink. When the drink is consumed, the person is rendered unconscious and is at great risk of attack. For more on date rape drugs click HERE.
Dangers of recreational ketamine use include;
Increased heart-rate
Depressed consciousness and breathing
Can lead to oxygen starvation to the muscles and brain
Vomiting
Temporary paralysis
Coma and eventually death
Ketamine is also known to be psychologically addictive, it is not uncommon to find users taking it daily.
Other problems:
Ketamine is often obtained in the comercial form Ketalar ®. This contains a preservative,benzethonium chloride, which is itself a potentially psychoactive ingredient.
Most brands of ketamine contain the same proportions of S(+) and R(-) stereoisomers (racemic ketamine), however, at least one brand has been found to contain only the S(+)stereoisomer. According to researchers, S(+) ketamine is more likely to suppress breathing and induce a faster loss of consciousness than R(-) ketamine.
Synthesis.
The synthetic pathway for ketamine is shown below:
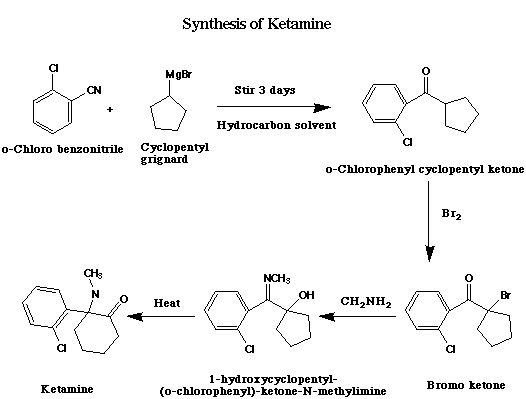
[Image taken from www.rhodium.ws without permission]
References.
![]() Back to Molecule of the Month page.
[DOI:10.6084/m9.figshare.5371207]
Back to Molecule of the Month page.
[DOI:10.6084/m9.figshare.5371207]