![]()
MENTHOL
(including the mint julep)
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Molecule of the Month August 2007
Also available: HTML version.
![]()
|
MENTHOL(including the mint julep)
Simon Cotton
Molecule of the Month August 2007
|
 |

Rhett Butler (Gone with the Wind) would not have been the man he was without his mint juleps.
 But what is a mint julep?
But what is a mint julep?Mint julep is the archetypal Southern drink, made from bourbon, ice, sugar and mint, particularly associated with the Kentucky Derby, a classic event for 3-year-old thoroughbreds, held over one and one-quarter miles at Churchill Downs racecourse on the first Saturday in May.
Menthol is a major component of mint. It is obtained by extracting mint by steam distillation, removing the water by drying, then chilling the resulting oil to crystallise the menthol. The best source is probably Mentha arvensis, Corn Mint, (see picture, below) whose oil is up to 85% (-)-menthol.


A mint plant - Mentha arvensis Lamb chop with mint sauce
Yes, the stuff made from chopped-up mint leaves in vinegar, with a dash of sugar. Just the thing for roast lamb.
 How is it made?
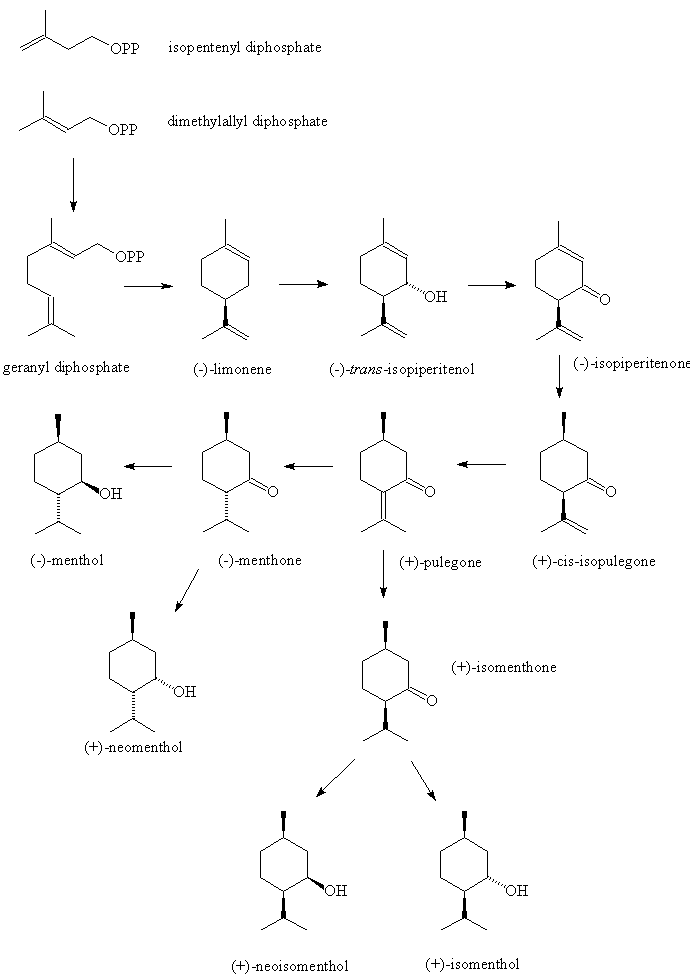
How is it made?The Japanese chemist Ryoji Noyori (picture, right), who shared in the Nobel Prize for Chemistry in 2001, developed rhodium-based catalysts for the synthesis of (-) menthol from myrcene. Such syntheses are now used commercially, with an enantiomeric excess of up to 100%.
There is a complicated pathway, involving a series of enzyme-catalysed reactions.
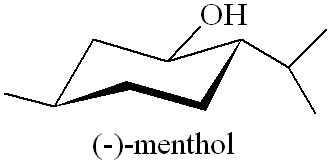
It's a colourless crystalline solid, m.p. 41 to 43°C, soluble in ethanol but insoluble in water. In nature it only occurs as (-) menthol, and its formal name is (1R,2S,5R)-2-isopropyl-5-methylcyclohexanol.
 |
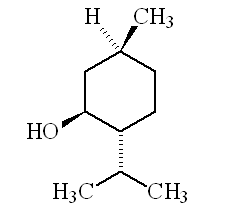 |
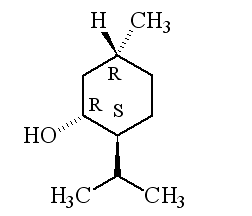
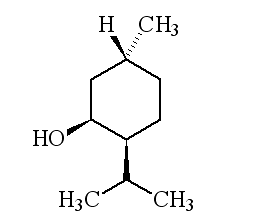
| 1R,3R,4S-(-)-menthol | 1S,3S,4R-(+)-menthol |
 Why is it called (-) menthol?
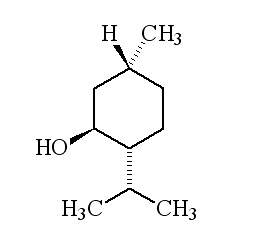
Why is it called (-) menthol?The molecule can exist as a pair of optical isomers, though natural menthol only contains the (-) form produced in biosynthesis. The 2-isopropyl-5-methylcyclohexanol molecule has three asymmetric (chiral) carbon atoms in its cyclohexane ring; it therefore occurs as four pairs of optical isomers. The other isomers are known as isomenthol, neomenthol and neoisomenthol. In menthol, all three bulky groups (OH, CH3 and CH(CH3)2) are in equatorial positions (see sketch, right), making menthol more stable than the other three isomers.
 |
 |
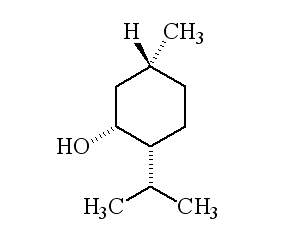 |
| 1R,3S,4S-(-)-menthol | 1R,3S,4R-(+)-menthol | 1R,3R,4R-(+)-menthol |
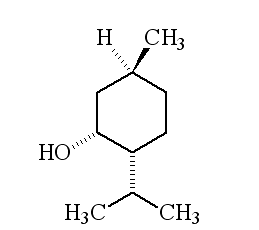 |
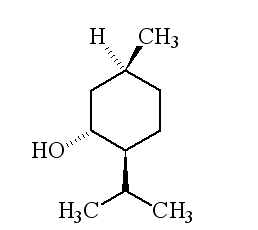 |
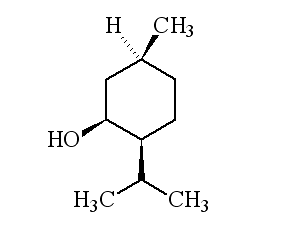 |
| 1S,3R,4R-(-)-menthol | 1S,3R,4S-(+)-menthol | 1S,3S,4S-(+)-menthol |
The two optical isomers (enantiomers) of menthol have identical chemical properties. They differ in those properties that depend upon the chiral carbon, like smell.
Although the atoms are connected in the same sequence, the molecules look different to a receptor.
(-) menthol can be described as "fresh, sweet, minty, cooling, refreshing". The (+) isomer is similar, but less minty, more herby, with "musty, bitter, phenolic and herbaceous notes", and is less refreshing. (-) menthol has also got about four times the cooling power of the (+) isomer.
Drug molecules work by binding to receptor sites on sensory neurons, cells that send messages to the brain. Capsaicin, the key molecule in chilli peppers, acts on the TRPV1 receptor, which is also sensitive to temperatures above 43°C, which is why chillis and curries are "hot". Menthol binds to the TRPM8 receptor, which is also sensitive to temperatures in a range ~8-28°C, hence the cooling effect of menthol.
When menthol binds to the TRPM8 receptor, it has the same effect upon the receptor as exposing it to "cool" temperatures, it activates a surface pore which causes calcium ions to flow into the cell. This causes an electrical change in the cell, which sends a message to the brain. And because the message is the same as when cooling causes the pore to open, the brain registers "cool" when menthol binds too.
Because of its "cooling" action, it is to be found in a wide range of medicines treating sore throats and mouth irritations. It is also used in treatments for minor aches and sprains, and in nasal decongestants. It is found in some oral hygiene products, including toothpaste and mouthwashes, and will combat "bad breath". Of course, it is present in a number of chewing gums. It was first added to cigarettes in the 1920s and 1930s (e.g. "Spud" and "Kool" brands) for both its flavour and also to reduce irritation, especially to the throat.
 And?
And?Don't forget Crème de menthe.
![]()
![]()